671576
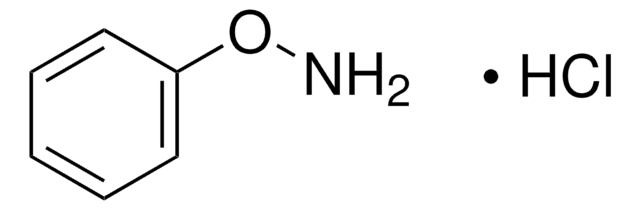
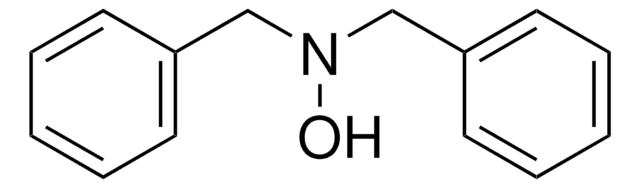
N-Phenylhydroxylamine
≥95.0%
Sinônimo(s):
N-Hydroxyaniline, N-Hydroxybenzenamine
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C6H7NO
Número CAS:
Peso molecular:
109.13
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
≥95.0%
Formulário
solid
pf
80-84 °C
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
ONc1ccccc1
InChI
1S/C6H7NO/c8-7-6-4-2-1-3-5-6/h1-5,7-8H
chave InChI
CKRZKMFTZCFYGB-UHFFFAOYSA-N
Categorias relacionadas
Aplicação
N-Phenylhydroxylamine can be used as a starting material for the synthesis of:
- 2-alkylindoles by treating with aliphatic terminal alkynes using gold catalyst via sequential 3,3-rearrangements and cyclodehydrations.
- Isoxazolidines by reacting with aldehydes and α, β-unsaturated aldehydes via a three-component one-pot catalytic reaction.
- Tetrahydro-1,2-oxazines by treating with an aldehyde and cyclopropane via homo 3+2 dipolar cycloaddition reaction.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Oral
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Au-catalyzed synthesis of 2-alkylindoles from N-arylhydroxylamines and terminal alkynes
Wang Y, et al.
Chemical Communications (Cambridge, England), 47(27), 7815-7817 (2011)
T P Bradshaw et al.
Free radical biology & medicine, 18(2), 279-285 (1995-02-01)
Previous studies have shown that incubation of rat red blood cells in vitro with phenylhydroxylamine (50-300 microM) induces rapid splenic sequestration of the red cells on reintroduction to isologous rats. EPR and the spin trapping agent, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), were utilized
Nilanjana Chowdhury et al.
Bioorganic & medicinal chemistry letters, 20(18), 5414-5417 (2010-08-21)
Photoinduced homolytic fission of nitrogen-oxygen bond in N,O-diacyl-4-benzoyl-N-phenylhydroxylamines using 310 nm UV light for 10 min produced acylaminyl and acyloxy radicals, which resulted in single strand cleavage of DNA at pH 7.0. Further the DNA cleaving ability of N,O-diacyl-4-benzoyl-N-phenylhydroxylamines found
A simple one-pot, three-component, catalytic, highly enantioselective isoxazolidine synthesis
Rios R, et al.
Tetrahedron Letters, 48(32), 5701-5705 (2007)
Christine S Olver et al.
Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis, 24(3), 273-278 (2012-12-12)
Carboxyheme and metheme states modulate hemostasis in humans and other species. Further, carbon monoxide and/or nitric oxide production increase in inflammatory disorders involving the gastrointestinal tract, with associated hypercoagulability or hypocoagulability. In particular, the horse suffers both thrombotic or coagulopathic
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica