558958
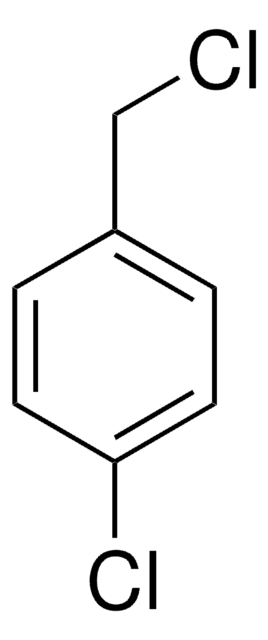
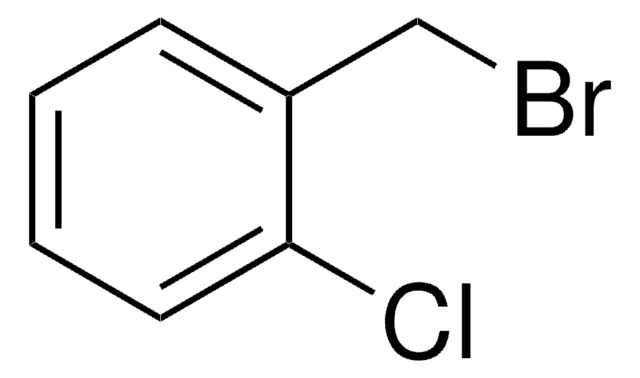
4-Chlorobenzyl bromide
97%
Sinônimo(s):
α-Bromo-p-chlorotoluene, 1-(Bromomethyl)-4-chlorobenzene, 1-Chloro-4-bromomethylbenzene, p-Chlorobenzyl bromide
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
ClC6H4CH2Br
Número CAS:
Peso molecular:
205.48
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
solid
pf
48-52 °C (lit.)
grupo funcional
bromo
chloro
cadeia de caracteres SMILES
Clc1ccc(CBr)cc1
InChI
1S/C7H6BrCl/c8-5-6-1-3-7(9)4-2-6/h1-4H,5H2
chave InChI
KQNBRMUBPRGXSL-UHFFFAOYSA-N
Descrição geral
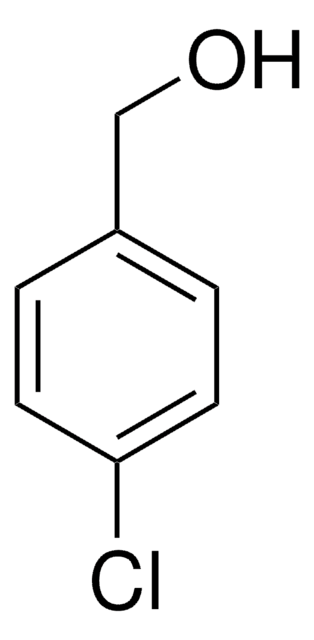
4-Chlorobenzyl bromide undergoes carbonylation in the presence of dimer of chloro(1,5-cyclooctadiene)rhodium(I) to yield the corresponding phenylacetic acid. It can be synthesized by reacting 4-chlorobenzyl alcohol with bromodimethylsulfonium bromide (BDMS) It can also be synthesized by refluxing a mixture of 4-chlorobenzaldehyde, chlorotrimethylsilane, 1,1,3,3-tetramethyldisiloxane and lithium bromide.
Aplicação
4-Chlorobenzyl bromide may be used to synthesize 1-(4-chlorobenzyl)-2-(pyrrolidin-1-yl-methyl)-1H-benzimidazole dihydrochloride
Embalagem
4-Chlorobenzyl bromide may be used to synthesize:
- 1-[1-[2-[(4-chlorobenzyl)thio]phenyl]vinyl]-1H-imidazole
- 6-[4-(4-chlorobenzyl)piperazinyl]chromane
- N,N′-bis(4-chlorobenzyl)piperazine
- N-(4-chlorobenzyl)piperazine
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Skin Corr. 1B
Código de classe de armazenamento
8A - Combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
6-(4-Benzylpiperazin-1-yl) benzodioxanes as selective ligands at cloned primate dopamine D 4 receptors.
Hodgetts KJ, et al.
Bioorganic & Medicinal Chemistry, 9(12), 3207-3213 (2001)
"Reduction of carbonyl compounds promoted by silicon hydrides under the influence of trimethylsilyl-based reagent"
Aizpurua.MJ, et al.
Canadian Journal of Chemistry, 64(12), 2342-2347 (1986)
Gazi S and Ananthakrishnan R
Royal Society of Chemistry Advances, 2(20), 7781- 7787 (2012)
Synthesis and antifungal activity of new 1-vinylimidazoles.
Ogata M, et al.
Journal of Medicinal Chemistry, 30(8), 1348-1354 (1987)
Synthesis of phenylacetic acids under rhodium-catalyzed carbonylation conditions.
Giroux A, et al.
Tetrahedron Letters, 41(40), 7601-7604 (2000)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica