543896
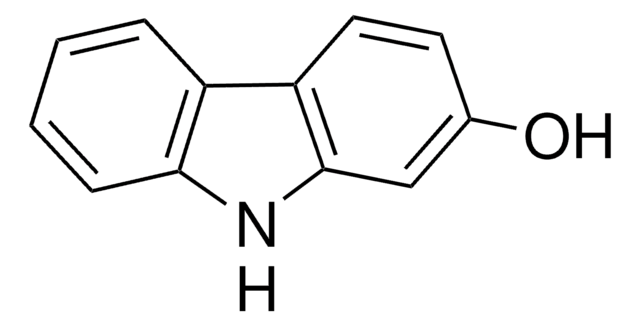
4-Hydroxycarbazole
95%
Sinônimo(s):
9H-Carbazol-4-ol
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C12H9NO
Número CAS:
Peso molecular:
183.21
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
95%
pf
169-173 °C (lit.)
cadeia de caracteres SMILES
Oc1cccc2[nH]c3ccccc3c12
InChI
1S/C12H9NO/c14-11-7-3-6-10-12(11)8-4-1-2-5-9(8)13-10/h1-7,13-14H
chave InChI
UEOHATPGKDSULR-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
4-Hydroxycarbazole can be obtained from 1,2,3,4-tetrahydro-4-oxocarbazole via dehydrogenation with freshly prepared Raney nickel.
Aplicação
4-Hydroxycarbazole may be used in the synthesis of the following:
It participates as an electron donor for the preparation of nonlinear optical (NLO) chromophores.
- 4-(2-bromoethoxy)-9H-carbazole
- 4-(3-bromopropoxy)-9H-carbazole
- 4-(4-bromobutoxy)-9H-carbazole
- 4-(5-bromopentyloxy)-9H-carbazole
- 4-(6-bromohexyloxy)-9H-carbazole
It participates as an electron donor for the preparation of nonlinear optical (NLO) chromophores.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Synthesis and characterization of novel electro-optic chromophores based on 4-hydroxycarbazole.
Zhou T, et al.
Materials Letters, 97, 117-120 (2013)
E A Dubois et al.
Journal of medicinal chemistry, 39(17), 3256-3262 (1996-08-16)
A new (radio)iodinated, beta-adrenoceptor ligand, (S)-(-)-4-[3-[(1,1-dimethyl-3-iodo-(2E)-propenyl)-amino]-2- hydroxypropoxy]carbazole (CYBL8E, 1), was prepared. 1 is an iodinated analogue of the high-affinity beta-adrenoceptor antagonist carazolol (2). The asymmetric synthesis was achieved in four steps starting from 4-hydroxycarbazole. The iodine-123-labeled form was obtained by
Pramod V Chavan et al.
Bioorganic chemistry, 85, 475-486 (2019-02-19)
A series of spirochromenocarbazole tethered 1,2,3-triazoles were synthesized via click chemistry based one-pot, five component reaction between N-propargyl isatins, malononitrile, 4-hydroxycarbazole, aralkyl halides and sodium azide using cellulose supported CuI nanoparticles (Cell-CuI NPs) as the heterogeneous catalyst. Antiproliferative activity of
Synthesis, biological evaluation, and molecular modeling of berberine derivatives as potent acetylcholinesterase inhibitors.
Huang L, et al.
Bioorganic & Medicinal Chemistry, 18(3), 1244-1251 (2010)
Michela Rosini et al.
Journal of medicinal chemistry, 51(15), 4381-4384 (2008-07-09)
Alzheimer's disease (AD) is a multifactorial syndrome with several target proteins contributing to its etiology. To confront AD, an innovative strategy is to design single chemical entities able to simultaneously modulate more than one target. Here, we present compounds that
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica