About This Item
Fórmula linear:
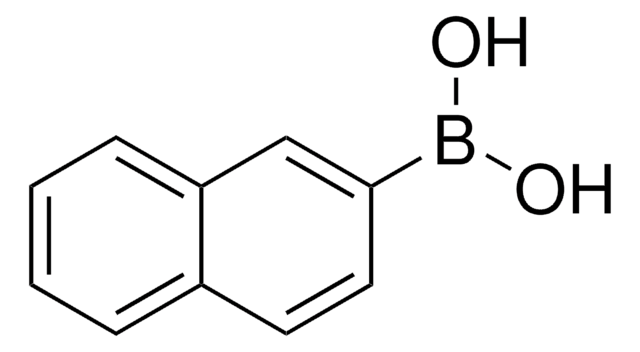
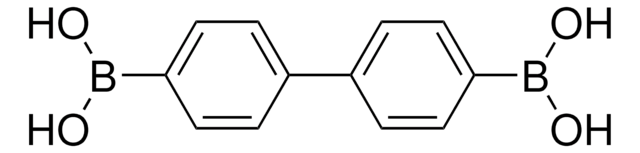
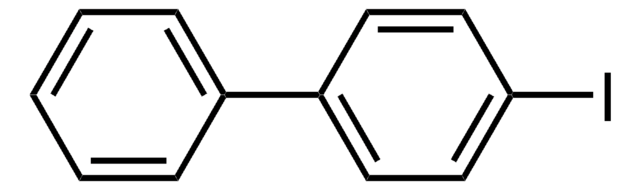
C6H5C6H4B(OH)2
Número CAS:
Peso molecular:
198.03
Número MDL:
Código UNSPSC:
12352103
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
≥95.0%
pf
232-245 °C (lit.)
grupo funcional
phenyl
cadeia de caracteres SMILES
OB(O)c1ccc(cc1)-c2ccccc2
InChI
1S/C12H11BO2/c14-13(15)12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,14-15H
chave InChI
XPEIJWZLPWNNOK-UHFFFAOYSA-N
Categorias relacionadas
Outras notas
Contains varying amounts of anhydride
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Kazuhiko Tsukagoshi et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 23(2), 227-230 (2007-02-14)
In our previous study, we proposed molecular recognition of mono- and disaccharides making use of the interaction between their diol groups and p-iodophenylboronic acid in capillary electrophoresis with a chemiluminescence detection system. Here, to extend our knowledge of molecular recognition
L J Kricka et al.
Journal of bioluminescence and chemiluminescence, 11(3), 137-147 (1996-05-01)
The enhancers 1,1'-biphenyl-4-yl boronic acid and 4-iodophenol act synergistically in the horseradish peroxidase-catalysed oxidation of luminol. This concentration-dependent effect reduces background, increases signal and hence improves signal/background for detection of peroxidase. The same type of synergistic effect was found when
Steven R Inglis et al.
Journal of medicinal chemistry, 52(19), 6097-6106 (2009-09-08)
Penicillin binding proteins (PBPs) catalyze steps in the biosynthesis of bacterial cell walls and are the targets for the beta-lactam antibiotics. Non-beta-lactam based antibiotics that target PBPs are of interest because bacteria have evolved resistance to the beta-lactam antibiotics. Boronic
Anna Minkkilä et al.
Journal of medicinal chemistry, 51(22), 7057-7060 (2008-11-06)
A series of commercial phenyl-, heteroaryl-, alkyl-, and alkenylboronic acids were evaluated for their FAAH and MGL inhibitory activities. The compounds were generally selective for FAAH, with IC50 in the nanomolar or low-micromolar range. Eight of these compounds inhibited MGL
Alessio Innocenti et al.
Bioorganic & medicinal chemistry letters, 19(10), 2642-2645 (2009-04-21)
Inhibition of the beta-carbonic anhydrases (CAs, EC 4.2.1.1) from the pathogenic fungi Cryptococcus neoformans (Can2) and Candida albicans (Nce103) with a series of aromatic, arylalkenyl- and arylalkylboronic acids was investigated. Aromatic, 4-phenylsubstituted- and 2-naphthylboronic acids were the best Can2 inhibitors
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica