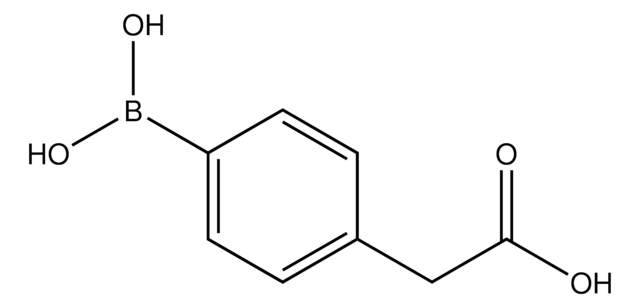
456764
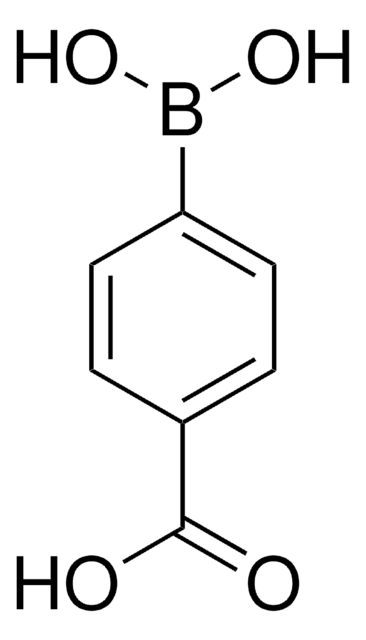
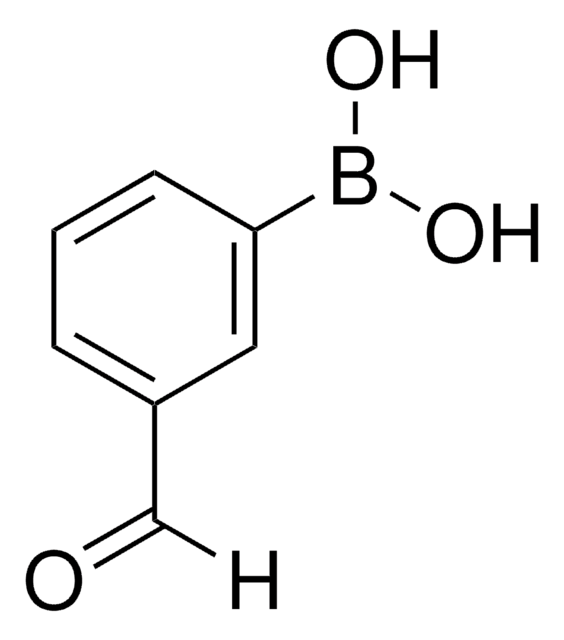
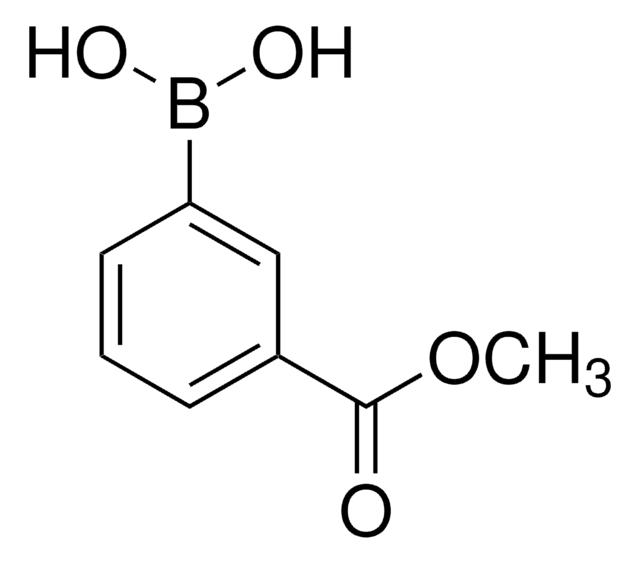
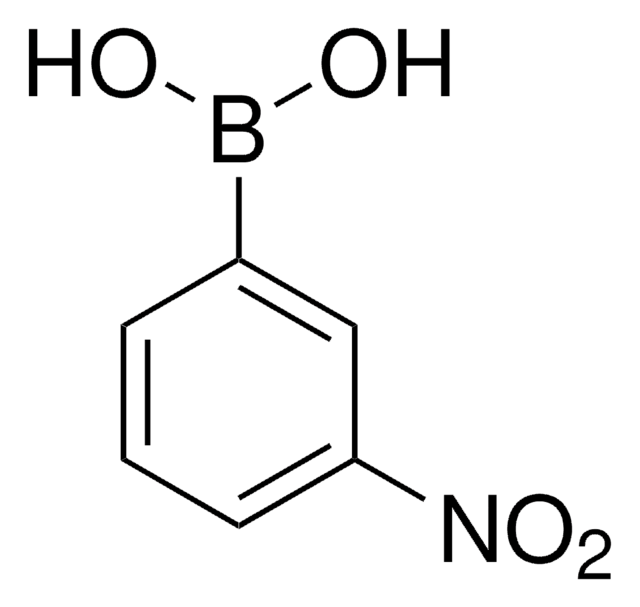
3-Carboxyphenylboronic acid
≥95%
Sinônimo(s):
μ-Carboxyphenylboronic acid, 3-(Dihydroxyborane)benzoic acid, 3-(Dihydroxyboryl)benzoic acid, 3-Boronobenzoic acid, 3-Carboxybenzeneboronic acid
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
HO2CC6H4B(OH)2
Número CAS:
Peso molecular:
165.94
Número MDL:
Código UNSPSC:
12352103
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
≥95%
pf
243-247 °C (lit.)
grupo funcional
carboxylic acid
cadeia de caracteres SMILES
OB(O)c1cccc(c1)C(O)=O
InChI
1S/C7H7BO4/c9-7(10)5-2-1-3-6(4-5)8(11)12/h1-4,11-12H,(H,9,10)
chave InChI
DBVFWZMQJQMJCB-UHFFFAOYSA-N
Categorias relacionadas
Aplicação
3-Carboxyphenylboronic acid can be used as a substrate in the preparation of:
- Biaryl derivatives by reacting with bromoaniline through the Suzuki-Miyaura coupling reaction.
- Boronic acid-functionalized block copolymer.
- 1H-Imidazo[1,2-a]quinoxaline derivatives.
Outras notas
Contains varying amounts of anhydride
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Synthesis of a phenylboronic acid-functionalized thermosensitive block copolymer and its application in separation and purification of vicinal-diol-containing compounds
Wang Y, et al.
Royal Society of Chemistry Advances, 6(85), 82309-82320 (2016)
Di Wu et al.
Acta biomaterialia, 96, 123-136 (2019-06-28)
Locoregional chemotherapy, especially using implantable hydrogel depots to sustainably deliver chemotherapeutics at tumor site, has shown great potential for improving antitumor efficacy and reducing systemic toxicity. However, the hydrogel applications are limited by some intrinsic constraints, especially the contradiction between
Jumin Yang et al.
Materials science & engineering. C, Materials for biological applications, 116, 111250-111250 (2020-08-19)
Various nanoparticles as drug delivery system provide significant improvements in the cancer treatment. However, their clinical success remains elusive in large part due to their inability to overcome both systemic and tumor tissue barriers. The nanosystems with nanoproperty-transformability (surface, size
Novel rhodamine dyes via Suzuki coupling of xanthone triflates with arylboroxins
Calitree, B. D.; Detty, M. R.
Synlett, 89-92 (2010)
New imidazo [1, 2-a] quinoxaline derivatives: synthesis and in vitro activity against human melanoma
Deleuze-Masquefa C, et al.
European Journal of Medicinal Chemistry, 44(9), 3406-3411 (2009)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 456764-10G | 4061832904573 |
| 456764-1G | 4061832341668 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica