325104
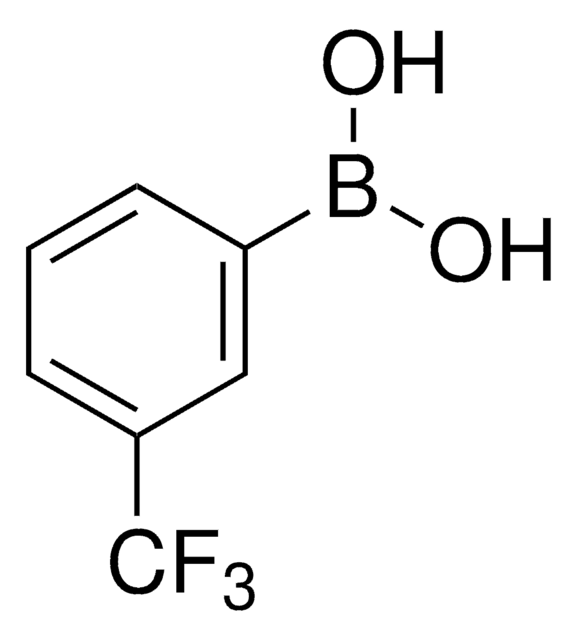
3-Nitrophenylboronic acid
≥97%
Sinônimo(s):
3-Nitrobenzeneboronic acid, m-Nitrobenzeneboronic acid, m-Nitrophenylboronic acid, NSC 401539, NSC 59739
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥97%
forma
powder
pf
284-285 °C (dec.) (lit.)
grupo funcional
nitro
cadeia de caracteres SMILES
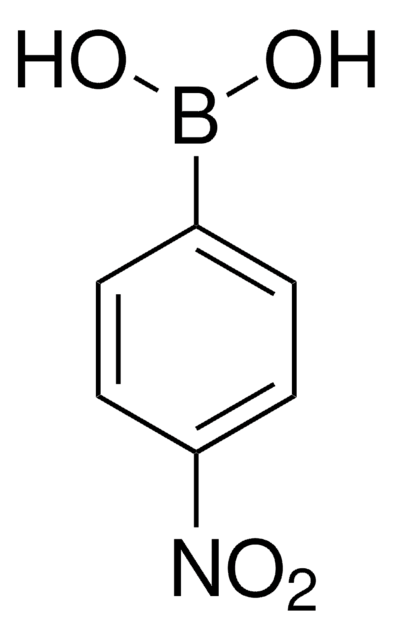
OB(O)c1cccc(c1)[N+]([O-])=O
InChI
1S/C6H6BNO4/c9-7(10)5-2-1-3-6(4-5)8(11)12/h1-4,9-10H
chave InChI
ZNRGSYUVFVNSAW-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Copper-catalyzed arylation
- Palladium-catalyzed decarboxylative coupling
- Suzuki-Miyaura cross-coupling
- Oxidative carbocyclization / arylation
- Addition to arylpropargyl alcohols
Additionally used as a reactant for synthesizing biologically active molecules such as:
- Inhibitors of angiogenesis
- Biaryl-olefins with antiproliferative activities
Outras notas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)