440914
2,2′-Azobis(2-methylpropionamidine) dihydrochloride
powder or granules, 97%
Sinônimo(s):
α,α′-Azodiisobutyramidine dihydrochloride, AAPH
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
powder or granules
t1/2
10 hr(56 °C)
pf
175-177 °C (lit.)
solubilidade
acetone, dioxane, methanol, ethanol, DMSO and water: soluble
cadeia de caracteres SMILES
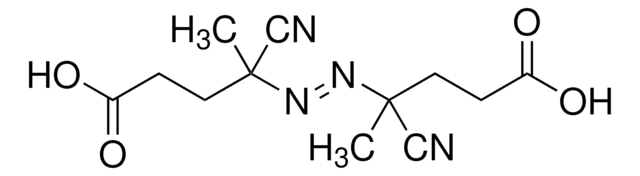
Cl.Cl.CC(C)(\N=N\C(C)(C)C(N)=N)C(N)=N
InChI
1S/C8H18N6.2ClH/c1-7(2,5(9)10)13-14-8(3,4)6(11)12;;/h1-4H3,(H3,9,10)(H3,11,12);2*1H/b14-13+;;
chave InChI
LXEKPEMOWBOYRF-QDBORUFSSA-N
Categorias relacionadas
Aplicação
Polymerization initiator for acrylic, vinyl and allyl monomers.
Características e benefícios
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Self-heat. 1 - Skin Sens. 1
Código de classe de armazenamento
4.2 - Pyrophoric and self-heating hazardous materials
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
We presents an article about a micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization. RAFT (Reversible Addition/Fragmentation Chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
Applying ARGET ATRP to the Growth of Polymer Brush Thin Films by Surface-initiated Polymerization
We presents an article about Copper(I)-mediated Living Radical Polymerization in the Presence of Pyridylmethanimine Ligands, and the emergence of living radical polymerization mediated by transition metal catalysts in 1995, which was a seminal piece of work in the field of synthetic polymer chemistry.
Protocolos
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Sigma-Aldrich presents an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
Sigma-Aldrich presents an article about the typical procedures for polymerizing via ATRP, which demonstrates that in the following two procedures describe two ATRP polymerization reactions as performed by Prof. Dave Hadddleton′s research group at the University of Warwick.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 440914-25G | 4061835515516 |
| 440914-100G | 4061835563098 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica