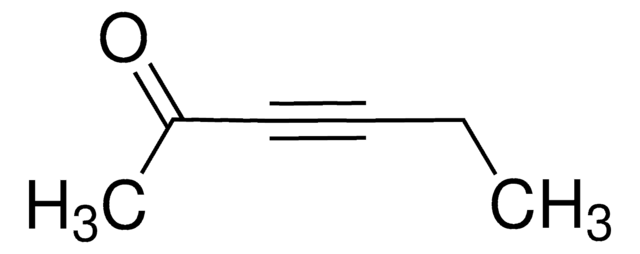About This Item
Fórmula linear:
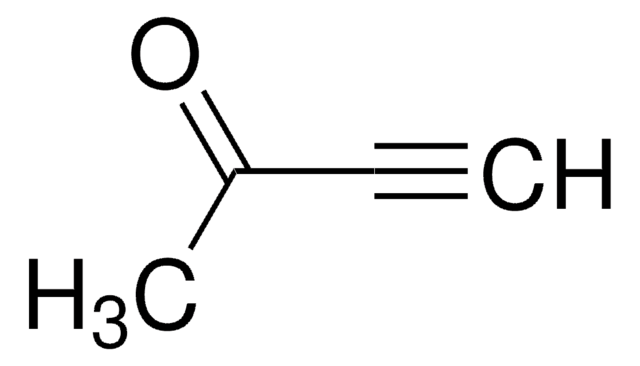
(CH3)3SiC≡CCOCH3
Número CAS:
Peso molecular:
140.26
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
liquid
índice de refração
n20/D 1.442 (lit.)
p.e.
156 °C (lit.)
densidade
0.854 g/mL at 25 °C (lit.)
grupo funcional
ketone
cadeia de caracteres SMILES
CC(=O)C#C[Si](C)(C)C
InChI
1S/C7H12OSi/c1-7(8)5-6-9(2,3)4/h1-4H3
chave InChI
NQEZDDPEJMKMOS-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
4-(Trimethylsilyl)-3-butyn-2-one is a ketone. Its asymmetric bioreduction to enantiopure {(S)-TMSBOL in various hydrophilic ionic liquid (ILs) solvent systems has been reported.
Aplicação
4-(Trimethylsilyl)-3-butyn-2-one (TMSB) has been used to investigate its asymmetric bioreduction to (S)-4-(trimethylsilyl)-3-butyn-2-ol {(S)-TMSBOL} by employing biocompatible water-immiscible ionic liquids (ILs). TMSB may be used for the synthesis of entecavir (BMS-200475).
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
82.4 °F - closed cup
Ponto de fulgor (°C)
28 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Javier Velasco et al.
The Journal of organic chemistry, 78(11), 5482-5491 (2013-05-18)
Entecavir (BMS-200475) was synthesized from 4-trimethylsilyl-3-butyn-2-one and acrolein. The key features of its preparation are: (i) a stereoselective boron-aldol reaction to afford the acyclic carbon skeleton of the methylenecylopentane moiety; (ii) its cyclization by a Cp2TiCl-catalyzed intramolecular radical addition of
Wen-Yong Lou et al.
BMC biotechnology, 9, 90-90 (2009-10-24)
Whole cells are usually employed for biocatalytic reduction reactions to ensure efficient coenzyme regeneration and to avoid problems with enzyme purification and stability. The efficiency of whole cell-catalyzed bioreduction is frequently restricted by pronounced toxicity of substrate and/or product to
Bo-Bo Zhang et al.
PloS one, 7(5), e37641-e37641 (2012-06-05)
Hydrophilic ionic liquids (ILs) were employed as green solvents to construct an IL-containing co-solvent system for improving the asymmetric reduction of 4-(trimethylsilyl)-3-butyn-2-one by immobilized Candida parapsilosis cells. Among 14 hydrophilic ILs examined, 1-(2'-hydroxyl)ethyl-3-methylimidazolium nitrate (C(2)OHMIM·NO(3)) was considered as the most
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 418676-5G | 4061837581533 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica