412562
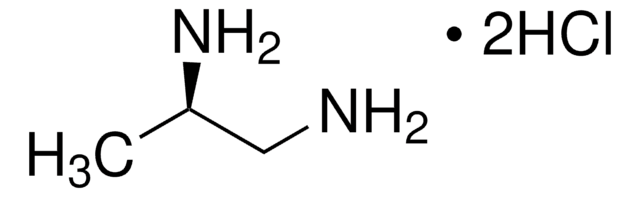
(S)-(−)-1,2-Diaminopropane dihydrochloride
99%
Sinônimo(s):
(S)-(−)-Propylenediamine dihydrochloride, (S)-1,2-Propanediamine dihydrochloride
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
CH3CH(NH2)CH2NH2·2HCl
Número CAS:
Peso molecular:
147.05
Beilstein:
5740936
Número MDL:
Código UNSPSC:
12352116
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
99%
atividade óptica
[α]22/D −4°, c = 20 in H2O
pf
227-229 °C (lit.)
grupo funcional
amine
cadeia de caracteres SMILES
Cl.Cl.C[C@H](N)CN
InChI
1S/C3H10N2.2ClH/c1-3(5)2-4;;/h3H,2,4-5H2,1H3;2*1H/t3-;;/m0../s1
chave InChI
AEIAMRMQKCPGJR-QTNFYWBSSA-N
Categorias relacionadas
Aplicação
(S)-(-)-1,2-Diaminopropane dihydrochloride may be used in the preparation of following chiral imidazoline derivatives, which show moderate α-adrenergic blocking activity:
- (S)-(-)-4-methyl-2-(1-naphthylmethyl)imidazoline hydrochloride
- (S)-(-)-2-benzyl-4-methylimidazoline picrate
- (S )-(-)-2-[(2,6-dichlorophenyl)imino]-4-methylimidazolidine hydrochloride
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Stereochemical studies of adrenergic drugs. Optically active derivatives of imidazolines.
Miller DD, et al.
Journal of Medicinal Chemistry, 19(12), 1382-1384 (1976)
Optically active derivatives of imidazolines: a-Adrenergic blocking properties.
Hsu FL, et al.
Journal of Medicinal Chemistry, 23(11), 1232-1235 (1980)
Chiral cyanide-bridged Mn(II)Mn(III) ferrimagnets, [Mn(II)(HL)(H2O)][Mn(III)(CN)6].2H2O (L = S- or R-1,2-diaminopropane): syntheses, structures, and magnetic behaviors.
Wakako Kaneko et al.
Journal of the American Chemical Society, 129(2), 248-249 (2007-01-11)
Keisuke Maruyoshi et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 15(7), 1618-1626 (2009-01-09)
Endogenous polyamines, represented by putrescine, spermidine, and spermine, are known to exert their physiological functions by interacting with polyanionic biomolecules such as DNA, RNA, adenosine triphosphate (ATP), and phospholipids. Very few examples of conformation analysis have been reported for these
S A M Fathi et al.
Journal of hazardous materials, 164(1), 133-137 (2008-09-10)
Bis(5-bromo-2-hydroxybenzaldehyde)-1,2-propanediimine is synthesized by the reaction of 5-bromo-2-hydroxybenzaldehyde and 1,2-diaminopropane in ethanol. This ligand is used as a modifier of octadecyl silica disks for preconcentration of trace amounts of copper(II) ions, followed by nitric acid elution and flame atomic absorption
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica