411744
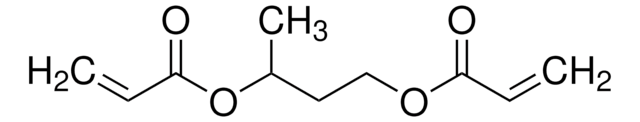
1,4-Butanediol diacrylate
technical grade, contains ~75 ppm hydroquinone as inhibitor
Sinônimo(s):
1,4-Bis(acryloyloxy)butane, Tetramethylene diacrylate
About This Item
Produtos recomendados
grau
technical grade
Ensaio
87%
forma
liquid
contém
~75 ppm hydroquinone as inhibitor
índice de refração
n20/D 1.456 (lit.)
pb
83 °C/0.3 mmHg (lit.)
densidade
1.051 g/mL at 25 °C (lit.)
temperatura de armazenamento
2-8°C
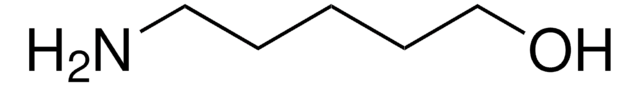
cadeia de caracteres SMILES
C=CC(=O)OCCCCOC(=O)C=C
InChI
1S/C10H14O4/c1-3-9(11)13-7-5-6-8-14-10(12)4-2/h3-4H,1-2,5-8H2
chave InChI
JHWGFJBTMHEZME-UHFFFAOYSA-N
Descrição geral
Aplicação
- As a precursor to synthesize joint-linker hydrogels with good mechanical strength and used as scaffold materials in bone tissue engineering as biomimetics for natural tissues and also in drug delivery systems.
- To prepare anti-fouling coating for dental composites.
- As a crosslinking agent to prepare hydrophobic acrylic intraocular lens(IOL) materials with reduced glistening.
- As a precursor to fabricate poly(β-amino ester) based solid polymer electrolytefilms for Li-ion batteries. BDDA enhances the ionic conductivity of theelectrolyte films.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1A
Código de classe de armazenamento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
>235.4 °F
Ponto de fulgor (°C)
> 113 °C
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica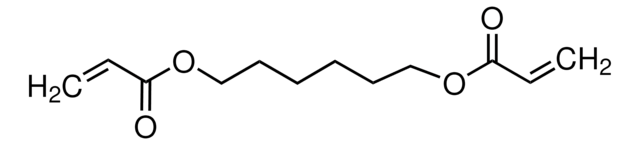
![1,8-Diazabiciclo[5,4,0]undec-7-eno 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)