399361
6-Oxoheptanoic acid
technical grade, 90%
Faça loginpara ver os preços organizacionais e de contrato
About This Item
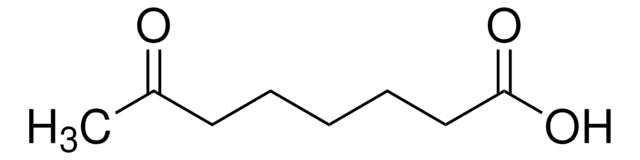
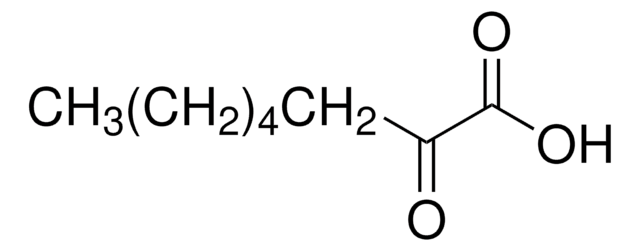
Fórmula linear:
CH3CO(CH2)4CO2H
Número CAS:
Peso molecular:
144.17
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
grau
technical grade
Ensaio
90%
Formulário
solid
p.e.
158-162 °C/9 mmHg (lit.)
pf
35-37 °C (lit.)
densidade
1.059 g/mL at 25 °C (lit.)
grupo funcional
carboxylic acid
ketone
cadeia de caracteres SMILES
CC(=O)CCCCC(O)=O
InChI
1S/C7H12O3/c1-6(8)4-2-3-5-7(9)10/h2-5H2,1H3,(H,9,10)
chave InChI
IZOQMUVIDMLRDC-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
6-Oxoheptanoic acid is a monocarboxylic acid with acyl functional group. Mass spectrometric characterization of 6-oxoheptanoic acid by electrospray ionization coupled to a triple quadrupole and TOF analyzer hybrid system has been reported.
Aplicação
6-Oxoheptanoic acid may be used in the following studies:
- As ketone linker used for the conjugation of hydrazide derivatives to proteins.
- Synthesis of N-(2-propynyl)-6-oxoheptanamide.
- Synthesis of adenosine triphosphate derivative.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Dam. 1 - Skin Corr. 1B
Código de classe de armazenamento
8A - Combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
235.4 °F - closed cup
Ponto de fulgor (°C)
113 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Mass spectrometric characterization of small oxocarboxylic acids and gas phase ion fragmentation mechanisms studied by electrospray triple quadrupole-MS/MS-TOF system and DFT theory.
Kanawati B, et al.
International Journal of Mass Spectrometry, 266(1), 97-113 (2007)
A Safavy et al.
Bioconjugate chemistry, 10(1), 18-23 (1999-01-20)
A procedure utilizing an activated ester approach for conjugation of unprotected hydroxamic acids to antibodies and peptides was recently reported. Here, an alternative method with advantages over the activated ester strategy is described. This protocol utilizes the hydrazone formation between
Synthesis of methylketone containing nucleoside triphosphates for RNA labelling
Trevisiol E, et al.
Tetrahedron, 56(35), 6501-6510 (2000)
Erik Selander et al.
Proceedings of the National Academy of Sciences of the United States of America, 112(20), 6395-6400 (2015-04-29)
Interactions among microscopic planktonic organisms underpin the functioning of open ocean ecosystems. With few exceptions, these organisms lack advanced eyes and thus rely largely on chemical sensing to perceive their surroundings. However, few of the signaling molecules involved in interactions
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica