391794
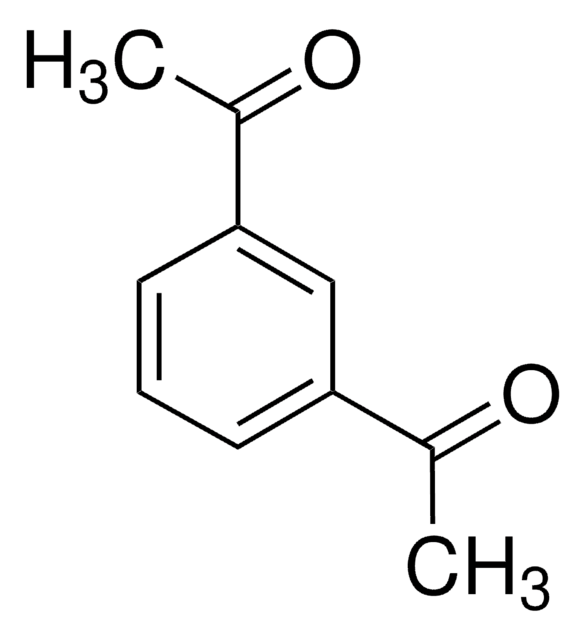
1,1′-(4,6-Dihydroxy-1,3-phenylene)bisethanone
99%
Sinônimo(s):
4,6-Diacetylresorcinol
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
(HO)2C6H2(COCH3)2
Número CAS:
Peso molecular:
194.18
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
99%
pf
178-180 °C (lit.)
grupo funcional
ketone
cadeia de caracteres SMILES
CC(=O)c1cc(C(C)=O)c(O)cc1O
InChI
1S/C10H10O4/c1-5(11)7-3-8(6(2)12)10(14)4-9(7)13/h3-4,13-14H,1-2H3
chave InChI
GEYCQLIOGQPPFM-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
1,1′-(4,6-Dihydroxy-1,3-phenylene)bisethanone (4,6-diacetylresorcinol, DAR) is a bifunctional carbonyl compound. Its synthesis by acetylating resorcinol in the presence of zinc chloride has been reported. The crystal structure of DAR has been studied.
Aplicação
1,1′-(4,6-Dihydroxy-1,3-phenylene)bisethanone (4,6-diacetylresorcinol, DAR) may be used in the synthesis of the following:
- Schiff base ligands
- hexadentate chalcogenated bisimine ligands
- 1,5-benzodiazepines
- ketimine of chitosan
- mannich bases
- hydrazone ligands
- thiosemicarbazone, semicarbazone and thiocarbohydrazone ligands
- binuclear cobalt(II) and copper(II) complexes
- europium (III) complexes
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Cahit Demetgül
Carbohydrate polymers, 89(2), 354-361 (2012-06-20)
In this study, a new chitosan derivative (ketimine) was synthesized by condensation of chitosan with 4,6-diacetylresorcinol (DAR) at heterogeneous medium. The ketimine derivative of chitosan (DAR-chitosan) was characterized by elemental (C, H, N), spectral (DR-UV-vis and FT-IR spectroscopy), structural (powder
Structure of 4, 6-diacetylresorcinol.
Kokila MK, et al.
Acta Crystallographica Section C, Crystal Structure Communications, 48(6), 1133-1134 (1992)
A Facile Synthesis of 2-Benzoyl-6-Hydroxy-3-Methyl-5-(2-Substituted-2, 3-Dihydro-1H-1,5-Benzodiazepin-4-YL) Benzo [b] Furans.
Reddy K, et al.
Synthetic Communications, 30(10), 1825-1836 (2000)
M Shebl et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 75(1), 428-436 (2009-12-08)
Mono- and binuclear VO(IV), Ce(III), Th(IV) and UO(2)(VI) complexes of thiosemicarbazone, semicarbazone and thiocarbohydrazone ligands derived from 4,6-diacetylresorcinol were synthesized. The structures of these complexes were elucidated by elemental analyses, IR, UV-vis, ESR, (1)H NMR and mass spectra as well
Magdy Shebl
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 70(4), 850-859 (2007-11-13)
A tetradentate N2O2 donor Schiff base ligand, H2L, was synthesized by the condensation of 4,6-diacetylresorcinol with benzylamine. The structure of the ligand was elucidated by elemental analyses, IR, 1H NMR, electronic and mass spectra. Reaction of the Schiff base ligand
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica