390704
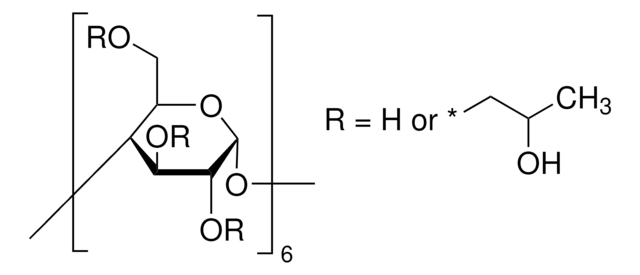
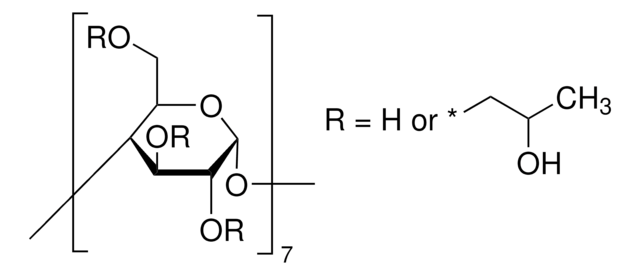
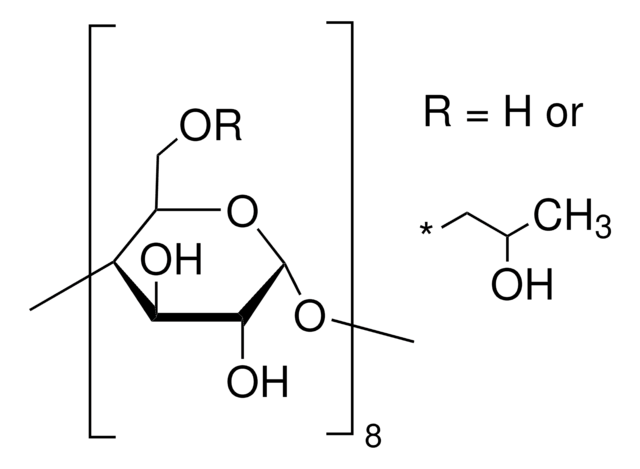
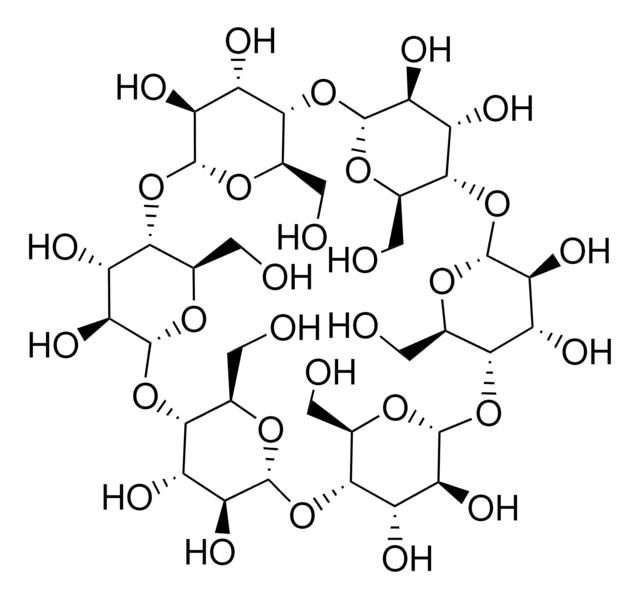
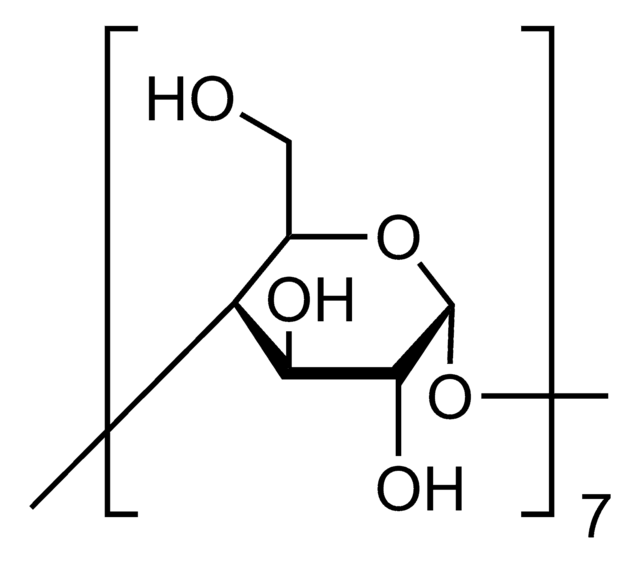
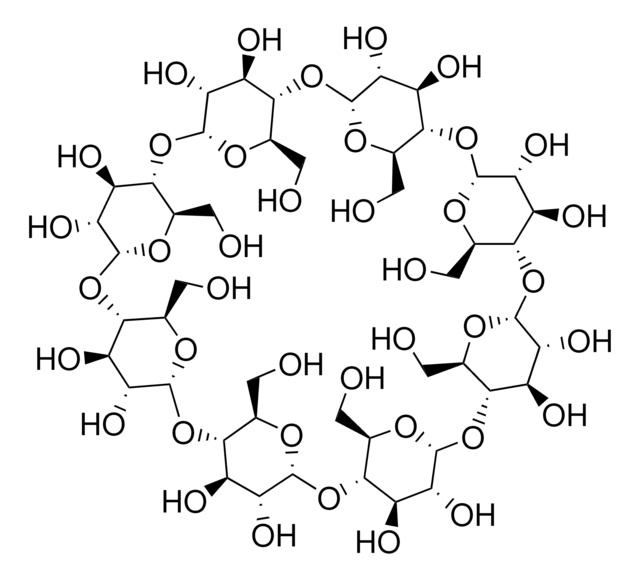
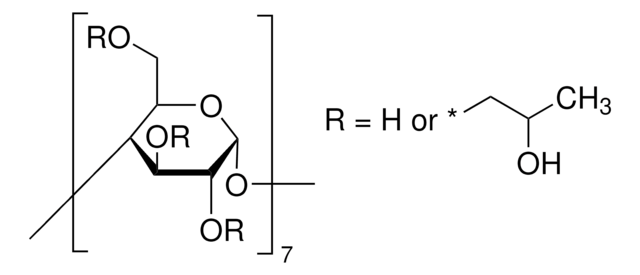
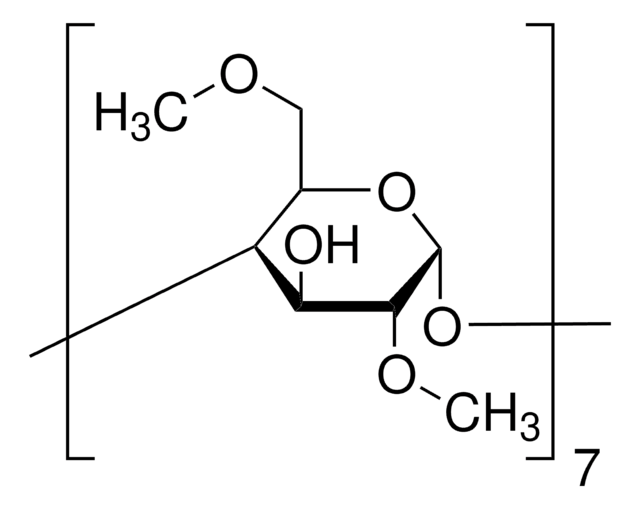
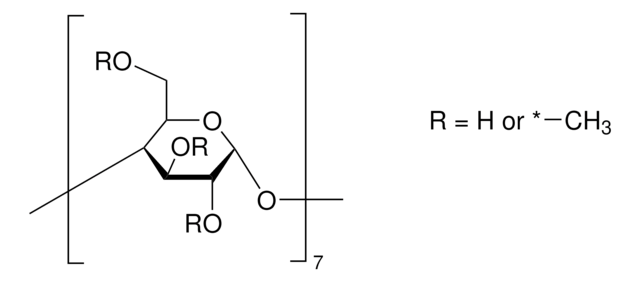
(2-Hydroxypropyl)-γ-cyclodextrin
extent of labeling: 0.6 molar substitution
Sinônimo(s):
HP-γ-CD, HPGCD, HGC
About This Item
Produtos recomendados
Formulário
powder
atividade óptica
[α]20/D +145°, c = 1 in H2O
peso molecular
average Mw ~1,580
Extensão da rotulagem
0.6 molar substitution
cadeia de caracteres SMILES
O1[C@@H]2O[C@H]([C@H](O[C@@H]3O[C@H]([C@H](O[C@@H]4O[C@H]([C@H](O[C@@H]5O[C@H]([C@H](O[C@@H]6O[C@H]([C@H](O[C@@H]7O[C@H]([C@H](O[C@@H]8O[C@H]([C@H]1[C@H]([C@@H]8O)O)COCC(O)C)[C@H]([C@@H]7O)O)CO)[C@H]([C@@H]6O)O)COCC(O)C)[C@H]([C@@H]5O)O)CO)[C@H]([C@@H]4O)
InChI
1S/C51H88O38/c1-14(56)8-73-11-21-42-29(64)36(71)50(81-21)85-40-19(6-54)79-48(34(69)27(40)62)89-44-23(13-75-10-16(3)58)82-51(37(72)30(44)65)86-41-20(7-55)78-47(33(68)26(41)61)88-43-22(12-74-9-15(2)57)80-49(35(70)28(43)63)84-39-18(5-53)76-45(31(66)24(39)59)83-38-17(4-52)77-46(87-42)32(67)25(38)60/h14-72H,4-13H2,1-3H3/t14?,15?,16?,17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-/m0/s1
chave InChI
ODLHGICHYURWBS-RYJYQAAZSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- As a mobile phase additive in the study of the host-guest interaction with organic low molecular mass compounds prior to their quantification using reversed phase-high performance liquid chromatography (RP-HPLC) technique.
- As a chiral surfactant for the analysis of econazole by micellar electrokinetic chromatography (MEKC).
- As an analytical standard for the determination of the analyte in biological samples by HPLC.
- As a chiral selector for the identification of propiconazole by MEKC.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica