366765
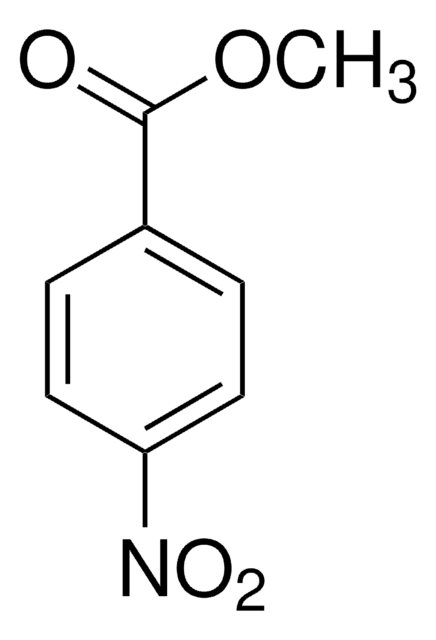
Methyl 2-methyl-3-nitrobenzoate
97%
Sinônimo(s):
Methyl 3-nitro-o-toluate
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
CH3C6H3(NO2)CO2CH3
Número CAS:
Peso molecular:
195.17
Beilstein:
1964020
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
solid
pf
62-65 °C (lit.)
grupo funcional
ester
nitro
cadeia de caracteres SMILES
COC(=O)c1cccc(c1C)[N+]([O-])=O
InChI
1S/C9H9NO4/c1-6-7(9(11)14-2)4-3-5-8(6)10(12)13/h3-5H,1-2H3
chave InChI
CRZGFIMLHZTLGT-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Methyl 2-methyl-3-nitrobenzoate may be used in the synthesis of:
- methyl indole-4-carboxylate
- 5-aminoisoquinolin-1(2H)-one
- 5-nitroisocoumarin
- substituted nitrostyrene benzoic acids, via reaction with aromatic aldehydes in the presence of DBU in DMSO
- 4-(hydroxymethyl)-1-tosylindole, via Batcho-Leimgruber modification of the Reissert indole synthesis
- [2-(4-fluorophenyl)-1H-indol-4-yl]-1-pyrrolidinylmethanone
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Synthesis of 2-Arylindole-4-Carboxylic Amides:[2-(4-Fluorophenyl)-1H-Indol-4-YL]-1-Pyrrolidinylmethanone.
Kuethe JT and Beutner GL.
Organic Syntheses, 92-104 (2009)
A N Brubaker et al.
Journal of medicinal chemistry, 29(8), 1528-1531 (1986-08-01)
Three 7-methyl-1,4-dioxa-7-azaspiro[4.5]decanes that contained either the benzyl, 3-indolylmethyl, or 4-indolylmethyl group at the 6-position were synthesized via alkylation of the pyrrolidine enamine of the key intermediate, ethyl 3-oxopiperidine-1-carboxylate. The spirodecane derivatives were evaluated for in vivo central and peripheral dopamine
The Chemistry of Indoles. XII. A Facile Route to 5-Nitroisocoumarins and Methyl Indole-4-carboxylate.
<BIG>Masanori S, et al. </BIG>
Chemical & Pharmaceutical Bulletin, 29(1), 249-253 (1981)
Preparation of 2-arylindole-4-carboxylic amide derivatives.
Kuethe JT and Davies LW.
Tetrahedron, 62(49), 11381-11390 (2006)
M C McDonald et al.
British journal of pharmacology, 130(4), 843-850 (2000-06-24)
Poly (ADP-ribose) synthetase (PARP) is a nuclear enzyme activated by strand breaks in DNA, which are caused inter alia by reactive oxygen species (ROS). Here we report on (i) a new synthesis of a water-soluble and potent PARP inhibitor, 5-aminoisoquinolinone
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica