358924
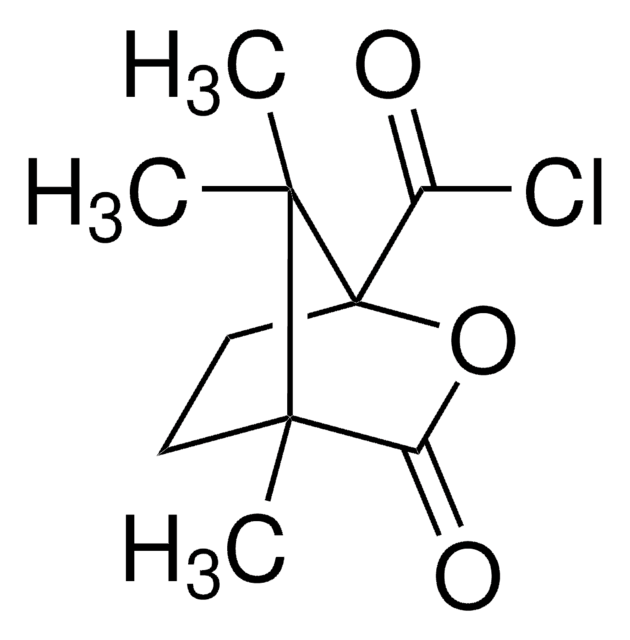
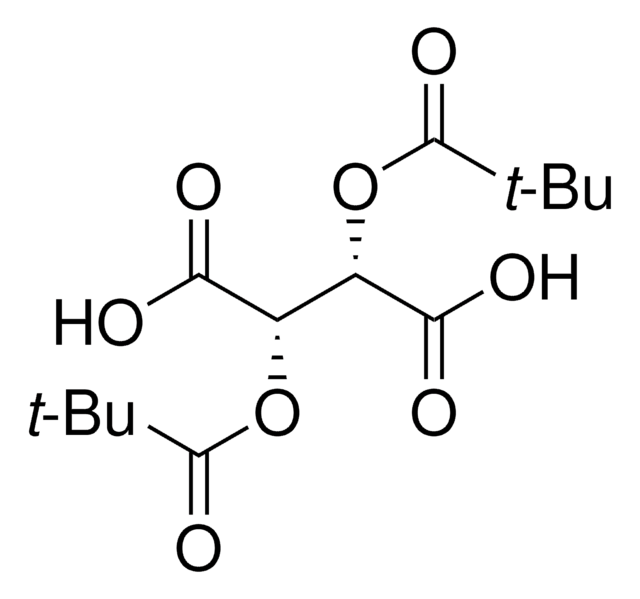
(+)-O,O′-Diacetyl-L-tartaric anhydride
97%
Sinônimo(s):
(+)-Diacetyl-L-tartaric anhydride
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C8H8O7
Número CAS:
Peso molecular:
216.14
Beilstein:
87315
Número CE:
Número MDL:
Código UNSPSC:
12352005
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
atividade óptica
[α]20/D +59°, c = 6 in acetone
pf
130-135 °C (lit.)
grupo funcional
anhydride
ester
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
CC(=O)O[C@@H]1[C@@H](OC(C)=O)C(=O)OC1=O
InChI
1S/C8H8O7/c1-3(9)13-5-6(14-4(2)10)8(12)15-7(5)11/h5-6H,1-2H3/t5-,6-/m1/s1
chave InChI
XAKITKDHDMPGPW-PHDIDXHHSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
(+)-O,O′-Diacetyl-L-tartaric anhydride is an HPLC derivatization reagent for UV/Vis detection. It is mainly employed as a reagent for the chiral derivatization of amino alcohols. It also reacts with alkanoamines in aprotic medium containing trichloroacetic acid and produces tartaric acid monoesters.
Aplicação
(+)-O,O′-Diacetyl-L-tartaric anhydride may be used as a chiral derivatizating agent in the following:
- determination of enantiomeric vigabatrin in mouse serum samples using ultra-high performance liquid chromatography-quadrupole-time-of-flight mass spectrometry (UHPLC-Q-TOF-M)
- determination of trantinterol in rat plasma by ultra performance liquid chromatography–electrospray ionization mass spectrometry (UPLC–MS/MS)
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Determination of enantiomeric vigabatrin by derivatization with diacetyl-l-tartaric anhydride followed by ultra-high performance liquid chromatography-quadrupole-time-of-flight mass spectrometry
Zhao J, et al.
Journal of Chromatography. B, Biomedical Sciences and Applications, 1040, 199-207 (2017)
D R Brocks et al.
Journal of pharmaceutical and biomedical analysis, 13(7), 911-918 (1995-06-01)
A stereospecific liquid chromatographic (LC) assay was developed for the quantification of the antimalarial drug, halofantrine, in human plasma. Following protein precipitation with acetonitrile, the enantiomers of halofantrine were extracted from human plasma using ammonium hydroxide and tert-butyl methyl ether-hexane.
D R Brocks et al.
Journal of chromatography, 581(1), 83-92 (1992-10-02)
(+/-)-Hydroxychloroquine (HCQ) is an antimalarial and anti-arthritic drug which is administered as the racemate. An accurate, precise and sensitive high-performance liquid chromatographic assay was developed for the determination of HCQ enantiomers in samples from human plasma, serum, whole blood, and
William M Oldham et al.
Bio-protocol, 6(16) (2017-06-03)
Two enantiomers of 2-hydroxyglutarate (2HG), L (L2HG) and D (D2HG), are metabolites of unknown function in mammalian cells that were initially associated with separate and rare inborn errors of metabolism resulting in increased urinary excretion of 2HG linked to neurological
W Lindner et al.
Journal of chromatography, 487(2), 375-383 (1989-02-24)
A sensitive high-performance liquid chromatographic method was developed for the stereoselective assay of (R)- and (S)-propranolol in human plasma. The method involves diethyl ether extraction of the drugs and a racemic internal standard, N-tert.-butylpropranolol, followed by derivatization of the compounds
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica