349801
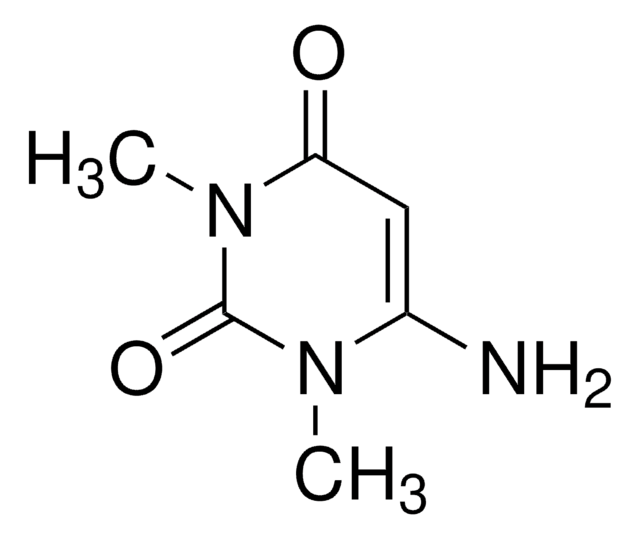
1,3-Dimethyluracil
99%
Sinônimo(s):
1,3-Dimethyl-2,4(1H,3H)-pyrimidinedione, 2,4-Dihydroxy-1,3-dimethylpyrimidine
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C6H8N2O2
Número CAS:
Peso molecular:
140.14
Beilstein:
124074
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
99%
forma
powder
pf
119-122 °C (lit.)
cadeia de caracteres SMILES
CN1C=CC(=O)N(C)C1=O
InChI
1S/C6H8N2O2/c1-7-4-3-5(9)8(2)6(7)10/h3-4H,1-2H3
chave InChI
JSDBKAHWADVXFU-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
1,3-Dimethyluracil is a pyrimidine derivative. Stability of the C6-centered carbanions derived from 1,3-dimethyluracil has been investigated in the gas phase and in DMSO and water solutions. The excited state structural dynamics of 1,3-dimethyluracil (DMU) in water and acetonitrile has been studied by resonance Raman spectroscopy. Crystal structure of 1,3-dimethyluracil has been reported. Ultraviolet irradiation of aqueous 1,3-dimethyluracil results in hydration of the 5:6 double bond of the uracil ring to form 1,3-dimethyl-6-oxy-hydrouracil.
Aplicação
1,3-Dimethyluracil is suitable reagent used to investigate the steady-state absorption and fluorescence spectra of uracil derivatives. It may be used in the preparation of 2,6-dihydroxynicotinamide.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Adam Gryff-Keller et al.
The journal of physical chemistry. A, 116(39), 9632-9638 (2012-09-14)
The practical utility of the method of retrieving the relaxation rate of a quadrupole nucleus via the scalar relaxation of the second kind (SC2) of an I = 1/2 spin nucleus has been considered once again. The study was motivated
Nicholas A Senger et al.
Tetrahedron, 69(26), 5287-5292 (2013-09-28)
The stabilities of the C6-centered carbanions derived from 1,3-dimethyluracil, N-methyl-2-pyridone, and N-methyl-4-pyridone were systematically investigated in the gas phase and in DMSO and water solutions. The stabilities of the carbanions in the gas phase and DMSO were directly measured through
Probing noncovalent interactions in biomolecular crystals with terahertz spectroscopy.
Thomas Kleine-Ostmann et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 9(4), 544-547 (2008-02-15)
H P Schuchmann et al.
International journal of radiation biology and related studies in physics, chemistry, and medicine, 50(6), 1051-1068 (1986-12-01)
Hydroxymethyl radicals .CH2OH, generated by the radiolysis of methanol (0.5 mol dm-3) in N2O-saturated aqueous solutions, were reacted with 1,3-dimethyluracil or 1,3-dimethylthymine (10(-3) mol dm-3). The products were identified and their G values determined. It has been concluded that in
Anna A Zadorozhnaya et al.
The journal of physical chemistry. A, 114(4), 2001-2009 (2010-01-09)
The electronic structure of 1,3-dimethyluracil and its dimer is characterized by ab initio calculations. The methylation eliminates the H-bonded isomers and allows one to focus on the pi-stacked manifold. In the neutral species, methylation increases the binding energy by 3-4
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica