326755
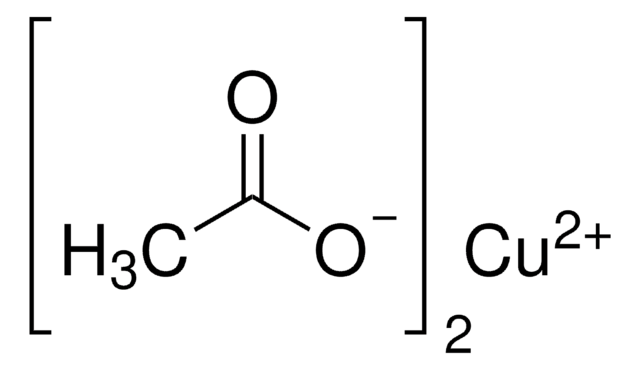
Copper(II) acetate
98%
Sinônimo(s):
Cupric acetate
About This Item
Produtos recomendados
grau
for analytical purposes
Nível de qualidade
densidade de vapor
6.9 (vs air)
Ensaio
98%
Formulário
powder or crystals
adequação da reação
reaction type: click chemistry
características do produto alternativo mais ecológico
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
categoria alternativa mais ecológica
, Aligned
cadeia de caracteres SMILES
CC(=O)O[Cu]OC(C)=O
InChI
1S/2C2H4O2.Cu/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
chave InChI
OPQARKPSCNTWTJ-UHFFFAOYSA-L
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Copper(II) acetate also known as cupric acetate, can be used as a catalyst in various processes in the field of greener chemistry. It is particularly useful in cross-coupling reactions, where it can promote the formation of carbon-carbon or carbon-heteroatom bonds, without the need for hazardous reagents or solvents
Aplicação
Copper-catalyzed reductive amination of aromatic and aliphatic ketones with anilines using environmental-friendly molecular hydrogen
Copper(II) acetate is used as a catalyst:
- In the N-arylation of α-amino esters with p-tolylboronic acid to synthesize biaryls via cross-coupling reactions
- In the the synthesis of substituted isoxazole derivatives
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Código de classe de armazenamento
8B - Non-combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
does not flash
Ponto de fulgor (°C)
does not flash
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 326755-25G | 4061826716458 |
| 326755-100G | 4061826716441 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica