320129
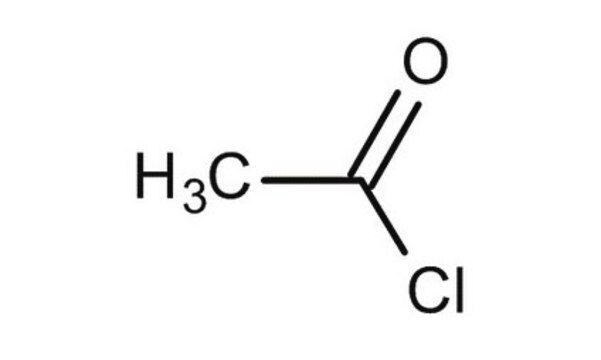
Acetyl chloride
reagent grade, 98%
Sinônimo(s):
Acetic acid chloride, Acetic chloride, Ethanoyl chloride
About This Item
Produtos recomendados
grau
reagent grade
Nível de qualidade
densidade de vapor
2.7 (vs air)
pressão de vapor
11.69 psi ( 20 °C)
32.33 psi ( 55 °C)
Ensaio
98%
Formulário
liquid
temperatura de autoignição
1353 °F
Lim. expl.
19 %
índice de refração
n20/D 1.389 (lit.)
p.e.
52 °C (lit.)
pf
−112 °C (lit.)
densidade
1.104 g/mL at 25 °C (lit.)
grupo funcional
acyl chloride
cadeia de caracteres SMILES
CC(Cl)=O
InChI
1S/C2H3ClO/c1-2(3)4/h1H3
chave InChI
WETWJCDKMRHUPV-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
- Synthesis, Characterization, and Evaluation of Thiazolidine Derivatives of Cysteine for Suppressing Eumelanin Production.: The study discusses the synthesis and evaluation of thiazolidine derivatives of cysteine, where acetyl chloride is used as a reagent, underscoring its importance in pharmaceutical intermediate development. (Amino et al., 2016).
- New URJC-1 Material with Remarkable Stability and Acid-Base Catalytic Properties.: This research introduces the new URJC-1 material, noting its stability and catalytic properties, with acetyl chloride being pivotal in the synthesis process, illustrating its role in material science and catalysis. (Leo et al., 2016).
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Perigos de suplementos
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
41.0 °F - closed cup
Ponto de fulgor (°C)
5 °C - closed cup
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 320129-1KG | 4061826695623 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica