287881
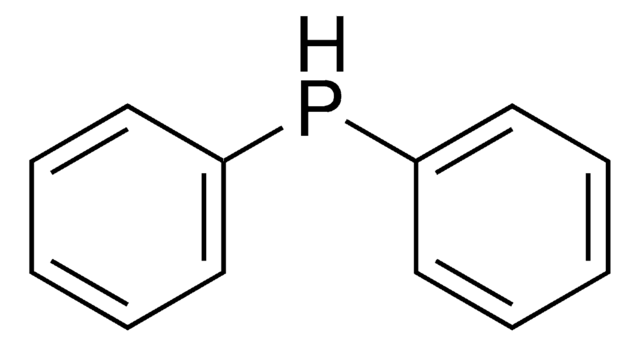
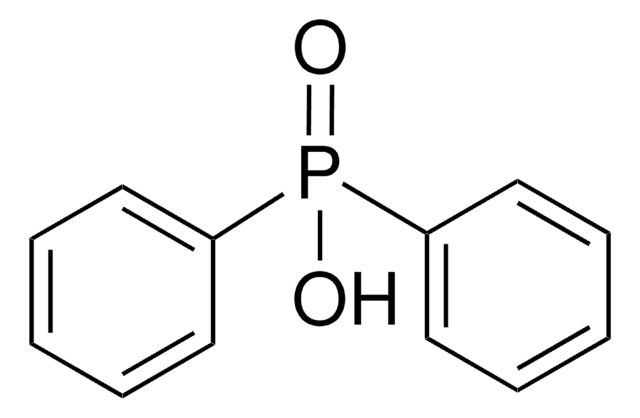
Diphenylphosphine oxide
97%
Sinônimo(s):
HPOPh2
About This Item
Produtos recomendados
Ensaio
97%
forma
solid
adequação da reação
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Hydrophosphonylations
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
pf
56-57 °C (lit.)
grupo funcional
phosphine
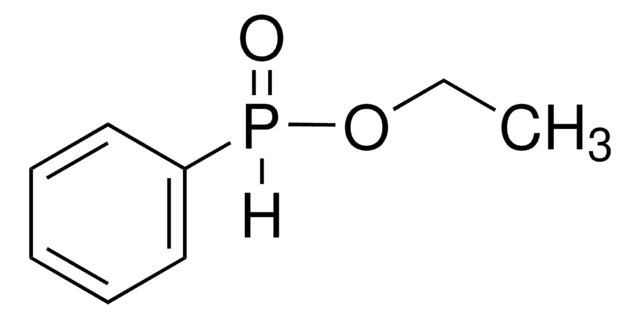
cadeia de caracteres SMILES
O=[PH](c1ccccc1)c2ccccc2
InChI
1S/C12H11OP/c13-14(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,14H
chave InChI
ASUOLLHGALPRFK-UHFFFAOYSA-N
Aplicação
It can also be used as a reactant to synthesize:
- Diphenyl(2-phenyl-2H-indazol-3-yl)phosphine oxide derivatives by phosphonylation of 2H-indazoles using rose bengal as a catalyst.
- Alkenyldiphenylphosphine oxides by hydrophosphinylation of terminal alkynes in the presence of transition metal catalysts.
- Diphenylphosphino-containing chiral ligands for asymmetric catalytic reactions via Pd-catalyzed coupling reaction with aryl triflates.
- Triarylphosphine oxides and Horner-Wittig reagents.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica