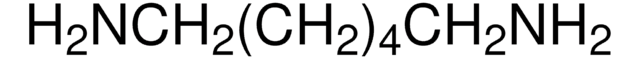247731
Hexamethylenediamine dihydrochloride
99%
Sinônimo(s):
1,6-Hexanediamine dihydrochloride
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
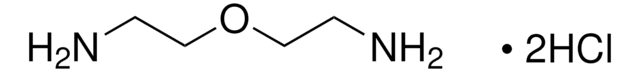
H2N(CH2)6NH2 · 2HCl
Número CAS:
Peso molecular:
189.13
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
99%
pf
256-257 °C (lit.)
solubilidade
water: freely soluble
grupo funcional
amine
cadeia de caracteres SMILES
Cl.Cl.NCCCCCCN
InChI
1S/C6H16N2.2ClH/c7-5-3-1-2-4-6-8;;/h1-8H2;2*1H
chave InChI
XMVQMBLTFKAIOX-UHFFFAOYSA-N
Descrição geral
Toxicity of hexamethylenediamine dihydrochloride has been investigated. Hexamethylenediamine dihydrochloride is also known as 1,6-diaminohexane dihydrochloride, 1,6-hexamethylenediamine dihydrochloride, 1,6- hexylenediamine dihydrochloride or 1,6-diamino-n-hexane dihydrochloride. Hexamethylenediamine dihydrochloride on fusion of 1:6-di-(N3-cyano-N1-guanidino)-hexane yields polymeric diguanides.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Charles Hebert
Toxicity report series, 24, 1-D8-1-D8 (1993-03-01)
1,6-Hexanediamine (HDA) is an aliphatic amine that is produced in large volumes in the United States. HDA is widely used as a corrosion inhibitor in lubricants and as an intermediate in the industrial synthesis of paints, resins, inks, and textiles.
850. Bisdiguanides having antibacterial activity.
Rose FL andSwain G.
Journal of the Chemical Society, 4422-4425 (1956)
Richard F G Fröhlich et al.
Carbohydrate research, 346(12), 1592-1598 (2011-06-08)
Two simple and reliably accessible intermediates, N-carboxypentyl- and N-aminohexyl-1-deoxy-D-galactonojirimycin were employed for the synthesis of a set of terminally N-dansyl substituted derivatives. Reaction of the terminal carboxylic acid of N-carboxypentyl-1-deoxy-D-galactonojirimycin with N-dansyl-1,6-diaminohexane provided the chain-extended fluorescent derivative. Employing bis(6-dansylaminohexyl)amine, the
Zhiming Zhang et al.
Inorganic chemistry, 47(17), 7615-7622 (2008-08-06)
The reaction between K 12[H 2P 2W 12O 48] and CuCl 2 in a NaCl aqueous solution assisted with organoamines (1,2-ethylenediamine (en), 1,6-hexamethylene diamine (hn), or both) leads to the isolation of three compounds: K 4Na 10[alpha 1-CuP 2W 17O
Wei-Chiang Chen et al.
ACS applied materials & interfaces, 1(8), 1821-1826 (2010-04-02)
Solvent microenvironments are formed around individual single-walled carbon nanotubes (SWNTs) by mixing SWNT suspensions with water-immiscible organic solvents. These microenvironments are used to encapsulate the SWNTs with the monomer sebacoyl chloride. Hexamethylene diamine is then injected into the aqueous phase
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica