247359
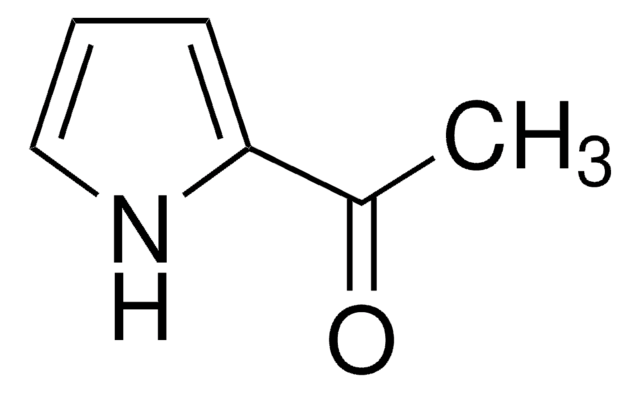
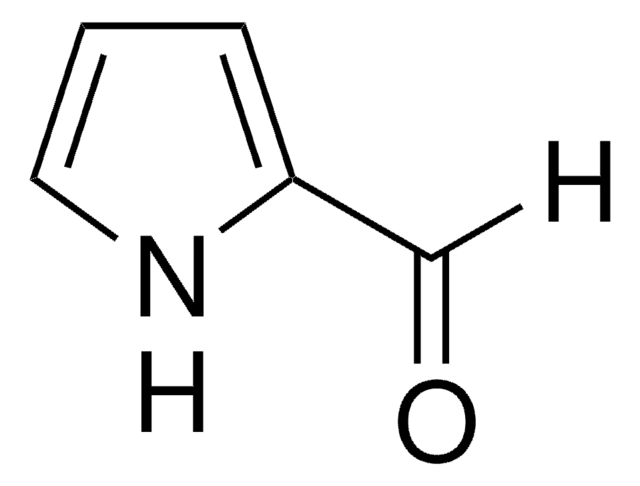
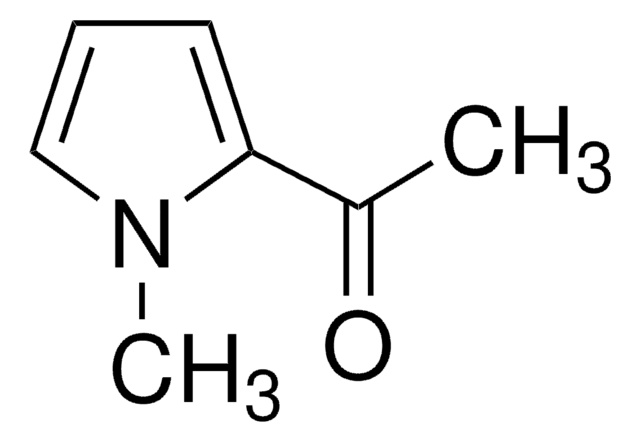
2-Acetylpyrrole
ReagentPlus®, 99%
Sinônimo(s):
Methyl 2-pyrrolyl ketone
About This Item
Produtos recomendados
linha de produto
ReagentPlus®
Ensaio
99%
p.e.
220 °C (lit.)
pf
88-93 °C (lit.)
grupo funcional
ketone
cadeia de caracteres SMILES
CC(=O)c1ccc[nH]1
InChI
1S/C6H7NO/c1-5(8)6-3-2-4-7-6/h2-4,7H,1H3
chave InChI
IGJQUJNPMOYEJY-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
Informações legais
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Protocolos
-Cymene; 2,5-Dimethylpyrrole; Acetoin, ≥96%, FCC, FG; 2,5-Dimethylpyrazine; 2,6-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Dimethylpyrazine; 4-Heptanone; 3-Ethylpyridine; 2,3,5-Trimethylpyrazine; Furfural; Pyrrole; Furfuryl acetate; Linalool; Linalyl acetate; 5-Methylfurfural; γ-Butyrolactone; 2-Acetyl-1-methylpyrrole; Furfuryl alcohol; 2-Acetylpyrrole; Pyrrole-2-carboxaldehyde
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica