About This Item
Fórmula linear:
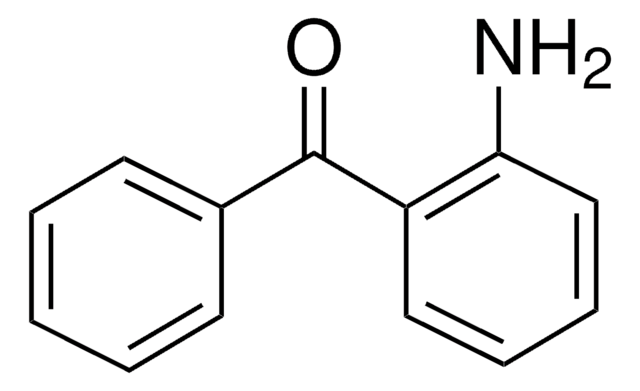
H2NC6H3(NO2)C(O)C6H5
Número CAS:
Peso molecular:
242.23
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
≥98%
Formulário
powder
pf
166-168 °C (lit.)
grupo funcional
ketone
nitro
phenyl
cadeia de caracteres SMILES
Nc1ccc(cc1C(=O)c2ccccc2)[N+]([O-])=O
InChI
1S/C13H10N2O3/c14-12-7-6-10(15(17)18)8-11(12)13(16)9-4-2-1-3-5-9/h1-8H,14H2
chave InChI
PZPZDEIASIKHPY-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
FT-IR and Raman spectra of 2-amino-5-nitrobenzophenone (ANBP) has been reported.
Aplicação
2-Amino-5-nitrobenzophenone was used in the synthesis of [5-(4-nitrophenyl)-2-furyl]acrylic acid substituted benzophenone (anti-malarial agent).
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Jochen Wiesner et al.
Bioorganic & medicinal chemistry letters, 13(3), 361-363 (2003-02-05)
We have developed the [5-(4-nitrophenyl)-2-furyl]acrylic acid substituted benzophenone 4g as a novel lead for anti-malarial agents. Here, we demonstrated that the acyl residue at the 2-amino group of the benzophenone core structure has to be a phenylacetic acid substructure substituted
V Balachandran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 118, 835-846 (2013-10-25)
The FT-IR and Raman spectra of 2-amino-5-nitrobenzophenone (ANBP) molecule have been recorded using Brucker IFS 66 V spectrometer in the range of 4000-100 cm(-1). The molecular geometry and vibrational frequencies in the ground state are calculated using the Hartree-Fock (HF)
P J Cox et al.
International journal of pharmaceutics, 194(2), 147-153 (2000-02-29)
The role of single crystal diffraction in the quantitative determination of polymorphism is demonstrated by the examination of three compounds. Two polymorphs were found for each of the compounds bis(2-nitrophenyl) trisulphide (1), 2-amino-5-nitrobenzophenone (2) and bis(2-nitrophenyl) sulphide (3). Only in
Koichi Saito et al.
Chemical & pharmaceutical bulletin, 69(3), 258-264 (2021-03-02)
The degradation behavior of eight benzodiazepines (BZPs): alprazolam, etizolam, diazepam, triazolam, nitrazepam (NZP), flunitrazepam (FNZ), bromazepam, and lorazepam, in artificial gastric juice was monitored by a LC/photodiode array detector (PDA) to estimate their pharmacokinetics in the stomach. For drugs that
T Inoue et al.
Journal of chromatography, 339(1), 163-169 (1985-04-12)
A method for the direct quantitative densitometry of nitrazepam and its main metabolites (7-aminonitrazepam, 7-acetamidonitrazepam and 2-amino-5-nitrobenzophenone) in urine was developed. The unchanged drug and its metabolites were extracted with benzene-dichloromethane (4:1), subjected to thin-layer chromatography, and determined by direct
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica
![BICYCLO[2.2.1]HEPT-5-ENE-2-CARBONITRILE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/423/632/9f9c4d06-10c1-4935-ac11-4f0e71373dd4/640/9f9c4d06-10c1-4935-ac11-4f0e71373dd4.png)