About This Item
Fórmula linear:
(O2N)2C6H3CH2OH
Número CAS:
Peso molecular:
198.13
Beilstein:
2054388
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
pf
88-91 °C (lit.)
grupo funcional
hydroxyl
nitro
cadeia de caracteres SMILES
OCc1cc(cc(c1)[N+]([O-])=O)[N+]([O-])=O
InChI
1S/C7H6N2O5/c10-4-5-1-6(8(11)12)3-7(2-5)9(13)14/h1-3,10H,4H2
chave InChI
GPHYIQCSMDYRGJ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
3,5-Dinitrobenzyl alcohol on reaction with p-toluenesulphonyl chloride yields 3,5-dinitrobenzyl p-toluenesulphonate.
Aplicação
3,5-Dinitrobenzyl alcohol was used in the synthesis of 3,5-bis((bezoxycarbonyl)imino)benzyl alcohol.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Ludmila Eisner et al.
Sensors (Basel, Switzerland), 19(18) (2019-09-13)
A sensor for trinitrotoluene (TNT) detection was developed by using a combination of optical micro-ring technology and a receptor coating based on molecularly imprinted sol-gel layers. Two techniques for deposition of receptor layers were compared: Airbrush technology and electrospray ionization.
K Funazo et al.
Journal of chromatography, 481, 211-219 (1989-11-03)
New UV-labelling agents have been synthesized, which are designed to convert monocarboxylic acids into their highly UV-absorbing derivatives for enhancement of the sensitivities of UV detection in high-performance liquid chromatography. The reagents are p-nitrobenzyl, 3,5-dinitrobenzyl and 2-(phthalimino)ethyl p-toluenesulphonates. Each has
Synthesis and characterization of hyperbranched polyurethanes prepared from blocked isocyanate monomers by step-growth polymerization.
Spindler R and Frechet JMJ.
Macromolecules, 26(18), 4809-4813 (1993)
Evon Powell et al.
International journal of nanomedicine, 2(3), 449-459 (2007-11-21)
The interaction of the important but often overdosed local anesthetic bupivacaine, its structural analogs 2,6-dimethylaniline, and N-methyl-2,6-dimethylacetanilide, and cocaine, with several electron deficient aromatic moieties were studied primarily by proton NMR and UV-visible spectroscopy. In solution, the anesthetic, its analogs
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica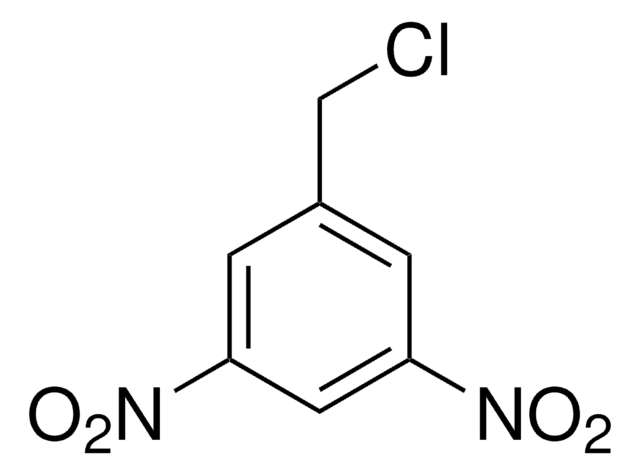

![1,8-Diazabiciclo[5,4,0]undec-7-eno 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)