About This Item
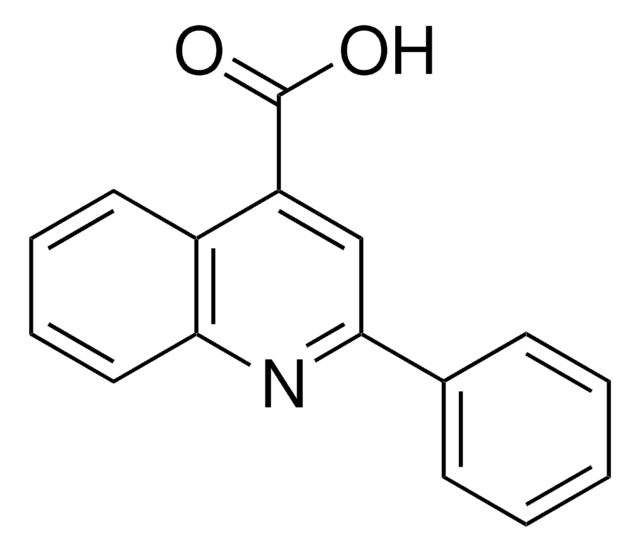
Fórmula empírica (Notação de Hill):
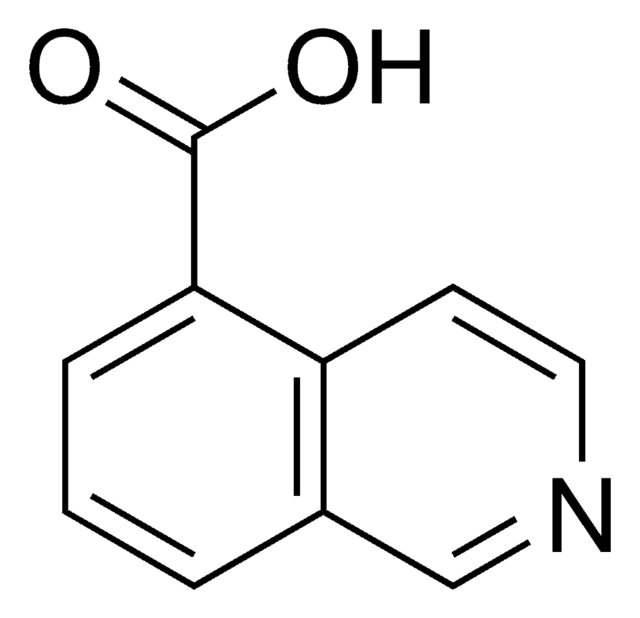
C10H7NO2
Número CAS:
Peso molecular:
173.17
Beilstein:
5224
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
pf
254-255 °C (lit.)
grupo funcional
carboxylic acid
cadeia de caracteres SMILES
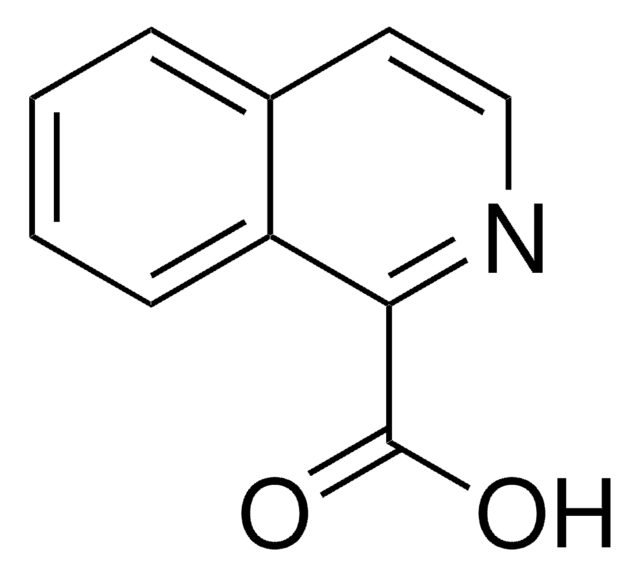
OC(=O)c1ccnc2ccccc12
InChI
1S/C10H7NO2/c12-10(13)8-5-6-11-9-4-2-1-3-7(8)9/h1-6H,(H,12,13)
chave InChI
VQMSRUREDGBWKT-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
4-Quinolinecarboxylic acid was used in the coupling reaction with diamine linker. A 4-quinolinecarboxylic acid analogue, brequinar sodium was used to inhibit dihydroorotate dehydrogenase and the de novo biosynthesis of pyrimidine.
Ações bioquímicas/fisiológicas
4-Quinolinecarboxylic acid showed anti-tumor activity against L1210 leukemia and B16 melanoma.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
D L Dexter et al.
Cancer research, 45(11 Pt 1), 5563-5568 (1985-11-01)
A novel, substituted 4-quinolinecarboxylic acid (NSC 339768) demonstrated antitumor activity against L1210 leukemia and B16 melanoma in the National Cancer Institute's Developmental Therapeutics Program. An extensive analogue synthesis program was initiated; over 200 derivatives were synthesized and tested for anticancer
[A case of Gaucher's disease treated with hydroxyphenylcinchoninic acid].
P DANIEL MARTINEZ et al.
Boletin medico del Hospital Infantil de Mexico, 8(2), 189-194 (1951-04-01)
A J Dobson et al.
Acta crystallographica. Section C, Crystal structure communications, 55 ( Pt 7), 1192-1195 (1999-08-13)
The previously undescribed title substance, C10H7NO2.-3H2O, crystallized in the centrosymmetric space group P1 with one zwitterionic organic molecule and three water molecules in the asymmetric unit. One N-H...O and six O-H...O hydrogen bonds are present in this structure, with donor-acceptor
Murugesan Dinakaran et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 4(5), 482-491 (2008-09-11)
Thirty four novel 7-fluoro/nitro-1,2-dihydro-5-oxo-8-(sub)-5H-thiazolo[3,2-a]quinoline-4-carboxylic acids were synthesized from 2,4-dichlorobenzoic acid and 2,4-dichloro-5-fluoroacetophenone by multi step reaction, evaluated for in vitro and in vivo antimycobacterial activities against Mycobacterium tuberculosis H37Rv (MTB), multi-drug resistant Mycobacterium tuberculosis (MDR-TB) and Mycobacterium smegmatis (MC2) and
He Huang et al.
The Journal of organic chemistry, 74(15), 5476-5480 (2009-07-04)
We developed a simple and convenient copper-catalyzed method for the synthesis of quinoline-2-carboxylate derivatives through sequential intermolecular addition of alkynes onto imines and subsequent intramolecular ring closure by arylation. The efficiency of this system allowed the reactions to be carried
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica