160660
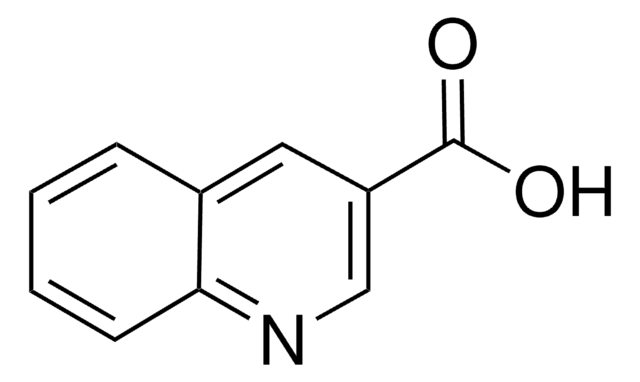
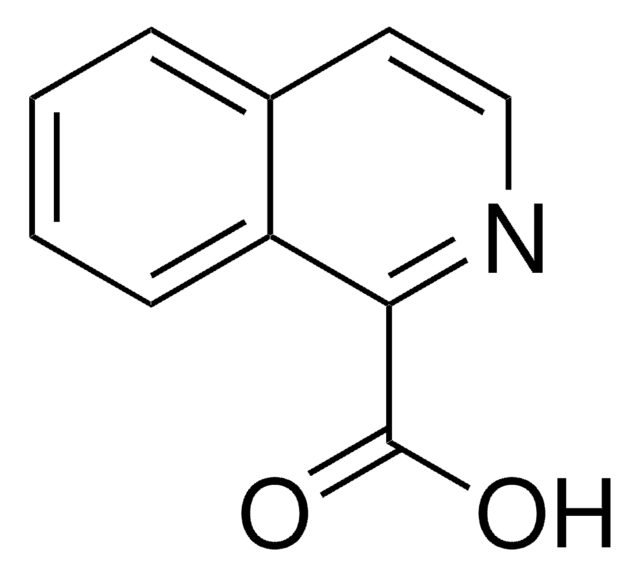
Quinaldic acid
98%
Sinônimo(s):
2-Quinolinecarboxylic acid
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C10H7NO2
Número CAS:
Peso molecular:
173.17
Beilstein:
126322
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
Formulário
solid
pf
156-158 °C (lit.)
grupo funcional
carboxylic acid
cadeia de caracteres SMILES
OC(=O)c1ccc2ccccc2n1
InChI
1S/C10H7NO2/c12-10(13)9-6-5-7-3-1-2-4-8(7)11-9/h1-6H,(H,12,13)
chave InChI
LOAUVZALPPNFOQ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Quinaldic acid is also referred as quinoline-2-carboxylic acid. Microwave-assisted preparation of substituted anilides of quinaldic acid has been reported. It inhibits the oxidation of pyruvate, α-ketoglutarate, glutamate and citrate in rat liver mitochondria. Quinaldic acid is a metabolite of tryptophan degradation and inhibits the gluconeogenesis in perfused livers.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
S Fetzner et al.
Biological chemistry Hoppe-Seyler, 374(6), 363-376 (1993-06-01)
Serratia marcecens 2CC-1 utilizes quinaldic acid (quinoline 2-carboxylic acid) as sole source of carbon, nitrogen and energy. Growth of strain 2CC-1 on quinaldic acid as well as on nicotinic acid and hypoxanthine was inhibited completely by the molybdate antagonist tungstate
Mode of action of hypoglycemic agents. 3. Studies on 5-methoxy indole-2-carboxylic acid and quinaldic acid.
J Reed et al.
The Journal of biological chemistry, 245(20), 5297-5303 (1970-10-25)
Total synthesis of thiostrepton, part 2: construction of the quinaldic acid macrocycle and final stages of the synthesis.
K C Nicolaou et al.
Angewandte Chemie (International ed. in English), 43(38), 5092-5097 (2004-09-17)
R M Epand
Medical hypotheses, 9(2), 207-213 (1982-08-01)
Ergothioneine is believed not to be synthesized by man but it accumulates to high concentrations in some mammalian cells as a result of dietary intake. Ergothioneine is known to chelate divalent metal ions with high affinity. Other substances which are
Stereocontrolled synthesis of the quinaldic acid macrocyclic system of thiostrepton.
K C Nicolaou et al.
Angewandte Chemie (International ed. in English), 41(11), 1937-1940 (2002-06-03)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica