144916
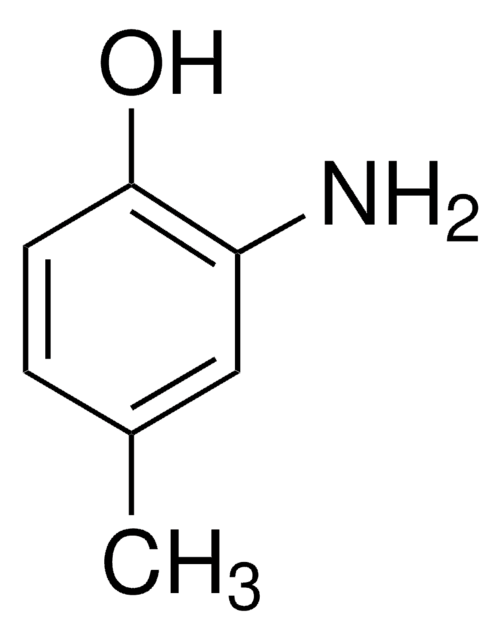
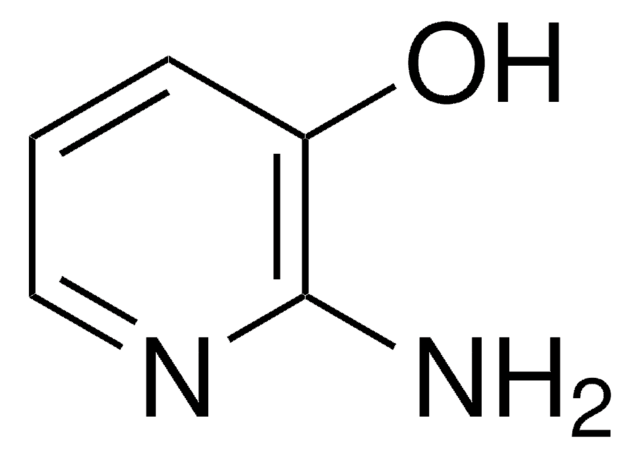
2-Amino-5-methylphenol
98%
Sinônimo(s):
2-Hydroxy-4-methylaniline, 4-Amino-3-hydroxytoluene, 6-Amino-m-cresol
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
H2NC6H3(CH3)OH
Número CAS:
Peso molecular:
123.15
Beilstein:
386144
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
forma
powder
pf
159-162 °C (lit.)
cadeia de caracteres SMILES
Cc1ccc(N)c(O)c1
InChI
1S/C7H9NO/c1-5-2-3-6(8)7(9)4-5/h2-4,9H,8H2,1H3
chave InChI
HCPJEHJGFKWRFM-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
2-Amino-5-methylphenol reacts with bovine hemoglobin to form 2-amino-4,4α-dihydro-4α-7-dimethyl-3H-phenoxazine-3-one, which inhibits the proliferation of Poliovirus in Vero cells. It is converted to dihydrophenoxazinone by purified human hemoglobin.
Aplicação
2-Amino-5-methylphenol was used in the synthesis of tridentate Schiff base ligand and novel non-metallocene catalysts with phenoxy-imine ligands.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Skin Sens. 1
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Antiviral activity of 2-amino-4,4alpha-dihydro-4alpha-7-dimethyl-3H-phenoxazine-3-one on poliovirus.
Akiko Iwata et al.
The Tohoku journal of experimental medicine, 200(3), 161-165 (2003-10-03)
2-Amino-4,4alpha-dihydro-4alpha-7-dimethyl-3H-phenoxazine-3-one (Phx), which was produced by the reaction of bovine hemoglobin with 2-amino-5-methylphenol, inhibited the proliferation of poliovirus in Vero cells between 0.25 microg/ml and 2 microg/ml with maximal antiviral acitivity at 1 microg/ml. These results suggest that Phx may
Synthesis,structural characterization and catalytic activity study of Mn(II), Fe(III), Ni(II), Cu(II) and Zn(II) complexes of quinoxaline-2-carboxalidine-2-amino-5-methylphenol: Crystal structure of the nickel (II) complex.
Sebastian M, et al.
Polyhedron, 29(15), 3014-3020 (2010)
The investigation of novel non-metallocene catalysts with phenoxy-imine ligands for ethylene (co-) polymerization.
Zhang X, et al.
Polymer International, 62(3), 419-426 (2012)
A Tomoda et al.
Bioorganic & medicinal chemistry letters, 11(8), 1057-1058 (2001-05-01)
A simple and rapid preparation method for a novel antitumor agent, 2-amino-4,4a-dihydro-4a,7-dimethyl-3H-phenoxazine-3-one (Phx) was described. The procedure included (1) the reaction of bovine hemolysates with 2-amino-5-methylphenol, (2) one-shot denaturation of hemoglobin and proteins by methanol, and removal of the denatured
A Tomoda et al.
Biochimica et biophysica acta, 1117(3), 306-314 (1992-10-27)
We found that 2-amino-5-methylphenol was converted to the dihydrophenoxazinone with a reddish brown color by purified human hemoglobin, lysates of human erythrocytes, and human erythrocytes. The reddish brown compound was identified as 2-amino-4,4 alpha-dihydro-4 alpha,7-dimethyl-3H-phenoxazin-3-one by the measurement of NMR
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica