122882
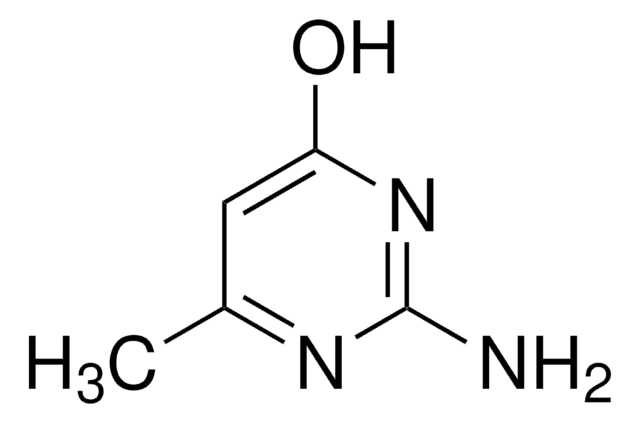
2-Amino-4-chloro-6-methylpyrimidine
97%
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C5H6ClN3
Número CAS:
Peso molecular:
143.57
Beilstein:
114297
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
forma
solid
pf
183-186 °C (lit.)
solubilidade
acetic acid: soluble 50 mg/mL, clear, colorless to faintly yellow
grupo funcional
chloro
cadeia de caracteres SMILES
Cc1cc(Cl)nc(N)n1
InChI
1S/C5H6ClN3/c1-3-2-4(6)9-5(7)8-3/h2H,1H3,(H2,7,8,9)
chave InChI
NPTGVVKPLWFPPX-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
2-Amino-4-chloro-6-methylpyrimidine is a nitification inhibitor.
Aplicação
2-Amino-4-chloro-6-methylpyrimidine was used to study the influence of chlorine substitution in pyrimidine ring on proton donor ability of amino group in 2-aminopyrimidine.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Influence of chlorine-substitution in pyrimidine ring on proton donor ability in H-bond and parameters of amino group of 2-amino pyrimidine.
Borisenko VE, et al.
Vibrational Spectroscopy, 37(1), 97-109 (2005)
T Jayavarthanan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 97, 811-824 (2012-08-21)
The solid phase FTIR and FT-Raman spectra of 2-amino-4-chloro-6-methylpyrimidine (2A4Cl6MP) have been recorded in the regions 400-4000 and 50-4,000 cm(-1), respectively. The spectra have been interpreted interms of fundamentals modes, combination and overtone bands. The structure of the molecule has
J A Hutter et al.
Biochemistry, 26(7), 1969-1973 (1987-04-07)
Thiaminase I from Bacillus thiaminolyticus strain Matsukawa et Misawa is completely and irreversibly inhibited by treatment with 4-amino-6-chloro-2-methylpyrimidine. Inhibition is a time-dependent first-order process, exhibiting a half-time of 4 h at an inhibitor concentration of 5 mM. A specific active-site-directed
Effects of nitrification inhibitors on denitrification of nitrate in soil.
Bremner JM andYeomans JC.
Biology and Fertility of Soils, 2(4), 173-179 (1986)
Christer B Aakeröy et al.
Pharmaceutics, 3(3), 601-614 (2011-01-01)
In the pharmaceutical industry, co-crystals are becoming increasingly valuable as crystalline solids that can offer altered/improved physical properties of an active pharmaceutical ingredient (API) without changing its chemical identity or biological activity. In order to identify new solid forms of
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica