704415
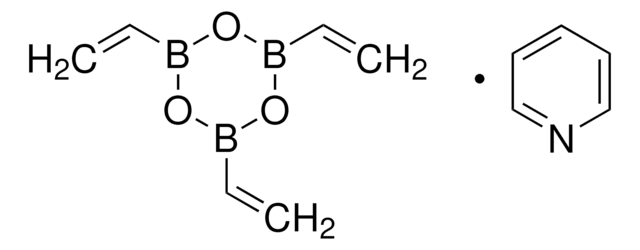
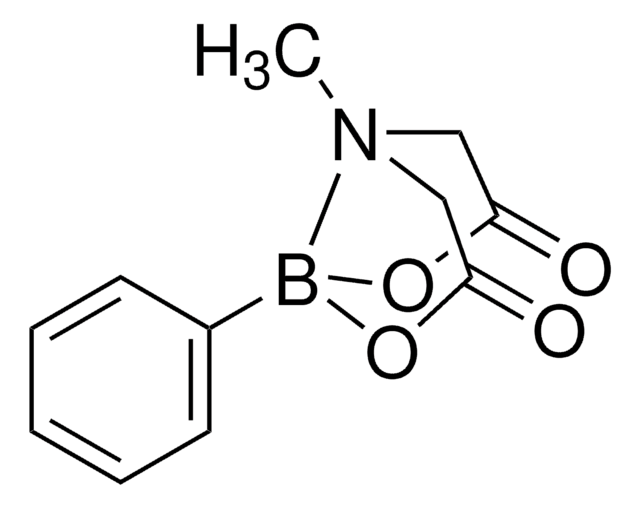
Vinylboronsäure-MIDAester
97%
Synonym(e):
6-Methyl-2-vinyl-1,3,6,2-dioxazaborocan-4,8-dion
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(4)
About This Item
Empirische Formel (Hill-System):
C7H10BNO4
CAS-Nummer:
Molekulargewicht:
182.97
MDL-Nummer:
UNSPSC-Code:
12352103
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Form
powder
mp (Schmelzpunkt)
152-156 °C
Lagertemp.
2-8°C
SMILES String
CN1CC(=O)OB(OC(=O)C1)C=C
InChI
1S/C7H10BNO4/c1-3-8-12-6(10)4-9(2)5-7(11)13-8/h3H,1,4-5H2,2H3
InChIKey
MGRQGYAVASCCAK-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
Vinylboronic acid MIDA ester, like other MIDA boronates, possesses the capacity for controlled, in situ slow-release of boronic acids under aqueous basic conditions allowing the cross-coupling of classically challenging substrates.
Anwendung
MIDA boronates as stable boronic acid surrogates for classically challenging cross-couplings
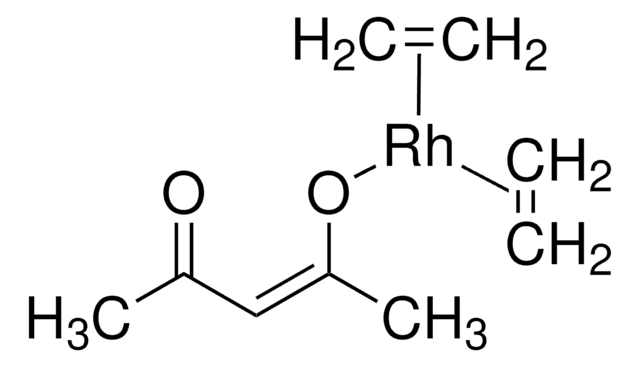
Suzuki Cross-Coupling with MIDA Boronates
Suzuki Cross-Coupling with MIDA Boronates
- Vinylboronic acid MIDA ester is an air and chromatographically stable boronic acid surrogate for Suzuki-Miyaura cross-coupling. It can also be used in Heck and oxidative Heck reactions as well as in olefin metathesis to provide the cross-coupled product.
- It is compatible with a wide range of common synthetic reagents that allows functionalization to synthesize structurally complex boronic acid surrogates.
- It undergoes cyclopropanation and epoxidation to yield corresponding MIDA cyclopropane and oxirane, respectively.
- It can be used as one of the major reagents for the scalable synthesis of potent cytotoxin, Leiodermatolide and for the total synthesis of (−)-Blepharocalyxin D.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Vinyl MIDA boronate: a readily accessible and highly versatile building block for small molecule synthesis.
Uno BE, et al.
Tetrahedron, 65(16), 3130-3138 (2009)
Synthesis, molecular editing, and biological assessment of the potent cytotoxin leiodermatolide.
Mailhol D, et al.
Journal of the American Chemical Society, 136(44), 15719-15729 (2014)
Synthesis of trans-2-(Trifluoromethyl) cyclopropanes via Suzuki reactions with an N-methyliminodiacetic acid boronate.
Duncton MA and Singh R.
Organic Letters, 15(17), 4284-4287 (2013)
Total Synthesis of (−)-Blepharocalyxin D and Analogues.
Cons BD, et al.
Organic Letters, 15(8), 2046-2049 (2013)
A general solution for unstable boronic acids: slow-release cross-coupling from air-stable MIDA boronates.
Knapp DM, et al.
Journal of the American Chemical Society, 131(20), 6961-6963 (2009)
Artikel
An article regarding MIDA-protected Boronate Esters.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.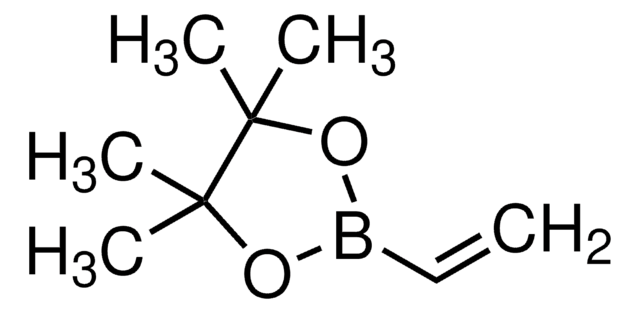
![[1,1′-Bis(diphenylphosphino)ferrocen]dichlorpalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)