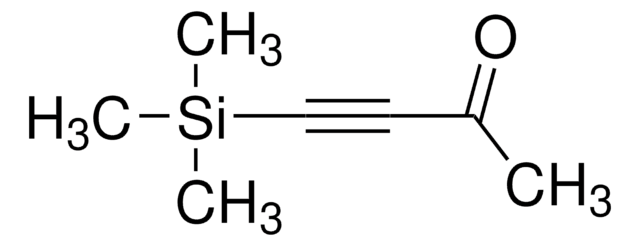Alle Fotos(3)
Wichtige Dokumente
637998
Vinylboronsäureanhydrid-Pyridin-Komplex
95%
Synonym(e):
2,4,6-Trivinylcyclotriboroxan-pyridin-Komplex
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Empirische Formel (Hill-System):
C6H9B3O3 · C5H5N
CAS-Nummer:
Molekulargewicht:
240.67
MDL-Nummer:
UNSPSC-Code:
12352103
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
95%
Form
solid
Lagertemp.
−20°C
SMILES String
c1ccncc1.C=Cb2ob(C=C)ob(C=C)o2
InChI
1S/C6H9B3O3.C5H5N/c1-4-7-10-8(5-2)12-9(6-3)11-7;1-2-4-6-5-3-1/h4-6H,1-3H2;1-5H
InChIKey
YLHJACXHRQQNQR-UHFFFAOYSA-N
Anwendung
Reagent used for
Reagent used for Preparation of
- Suzuki-Miyaura cross-coupling
- Stereoselective synthesis via Palladium-catalyzed carboamination
- Alkyl-connected 2-amino-6-vinylpurine (AVP) crosslinking agent to cytosine base in RNA
- Kaiser oxime resin-derived palladacycle as a recoverable polymeric precatalyst in Suzuki-Miyaura cross-coupling reactions in aqueous media
- Kinetic resolution of phosphoryl and sulfonyl esters of binaphthol derivatives via Pd-catalyzed alcoholysis of their vinyl ethers
- Stereoselective isomerization of N-allyl aziridines into Z-enamines by using rhodium hydride catalysis
- Kinetic resolution of axially chiral biaryl derivatives via palladium/chiral diamine ligand-catalyzed alcoholysis
- Transition metal-catalyzed alkenylation of aziridines, cycloaddition and thermal rearrangement reactions
- Intramolecular Heck reaction strategy for synthesis of functionalized tetrahydroanthracenes
Reagent used for Preparation of
- BACE-1 inhibitors and SAR of cyclic sulfone hydroxyethylamines
- Distorted spiropentanes
- Small molecule bradykinin B2 receptor antagonists in angioedema therapy
- Enol Ethers
- Styryl cyclobutanone
Signalwort
Warning
H-Sätze
P-Sätze
Gefahreneinstufungen
Eye Irrit. 2
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
176.0 °F - closed cup
Flammpunkt (°C)
80 °C - closed cup
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Kuan-Jen Su et al.
The Journal of organic chemistry, 75(21), 7494-7497 (2010-10-14)
Tetravinylbenzene 4 was prepared in nearly quantitative yield from commercially available tetrabromobenzene; the improved, one-step procedure now employs Suzuki-Miyaura cross-coupling conditions. Intermolecular cyclopropanation of 4 with dibromocarbene gave a series of gem-dibromide adducts. Intramolecular cyclopropanation of monoadduct 5, putatively by
Masahiro Murakami et al.
Organic letters, 7(10), 2059-2061 (2005-05-07)
Two structurally distinct carbocycles were selectively obtained by the reactions of 2-(o-styryl)cyclobutanones promoted by ytterbium salts. Treatment of the cyclobutanones with YbCl(3) in 1,4-dioxane at 100 degrees C afforded 2-(2-chloroethyl)naphthalenes. On the other hand, the reaction with Yb(OTf)(3) in chlorobenzene
Product subclass 3: enol ethers
Milata, V.; Radl, S.; Voltrova, S.
Sci. Synth., 32, 589-756 (2008)
Derek S Tsang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(3), 886-894 (2007-11-10)
In the presence of rhodium(I) hydride catalysts, tertiary N-allylamines are known to isomerise into E enamines. In contrast, we have recently found that N-allylaziridines isomerise to form Z enamines. On the basis of literature data, the most likely mechanism of
Kinetic resolution of phosphoryl and sulfonyl esters of 1,1'-bi-2-naphthol via Pd-catalyzed alcoholysis of their vinyl ethers
Sakuma, T.; et al.
Tetrahedron, 19, 1593-1599 (2008)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.