C0378
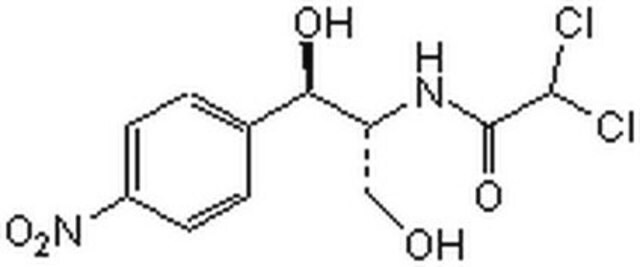
Chloramphenicol
≥98% (HPLC)
Synonym(s):
D-(−)-threo-2,2-Dichloro-N-[β-hydroxy-α-(hydroxymethyl)-β-(4-nitrophenyl)ethyl]acetamide, D-(−)-threo-2-Dichloroacetamido-1-(4-nitrophenyl)-1,3-propanediol, D-threo-2,2-Dichloro-N-[β-hydroxy-α-(hydroxymethyl)-4-nitrophenethyl]acetamide, Chloromycetin
About This Item
Recommended Products
Quality Level
assay
≥98% (HPLC)
form
powder or crystals
pKa
5.5
mp
149-153 °C (lit.)
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mycobacteria
mycoplasma
mode of action
protein synthesis | interferes
storage temp.
room temp
SMILES string
OC[C@@H](NC(=O)C(Cl)Cl)[C@H](O)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C11H12Cl2N2O5/c12-10(13)11(18)14-8(5-16)9(17)6-1-3-7(4-2-6)15(19)20/h1-4,8-10,16-17H,5H2,(H,14,18)/t8-,9-/m1/s1
InChI key
WIIZWVCIJKGZOK-RKDXNWHRSA-N
Gene Information
human ... CYP1A2(1544)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Chloramphenicol has been used as a bacteriostatic non potent antibiotic. It has been used for the selection/growth of positive bacterial cells.
Biochem/physiol Actions
Mode of Resistance: Use of chloramphenicol acetyltransferase will acetylate the product and inactivate it.
Antimicrobial Spectrum: This is a broad spectrum antibiotic against gram-positive and gram-negative bacteria, and is used mainly for ophthalmic and veterinary purposes.
Caution
Preparation Note
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Repr. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
This procedure applies to products that have a specification for the enzymatic activity of chloramphenicol acetyltransferase.
A highly selective and sensitive analytical method was developed for chloramphenicol using a QuEChERS type sample preparation approach and LC-MS/MS detection.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service