V8879
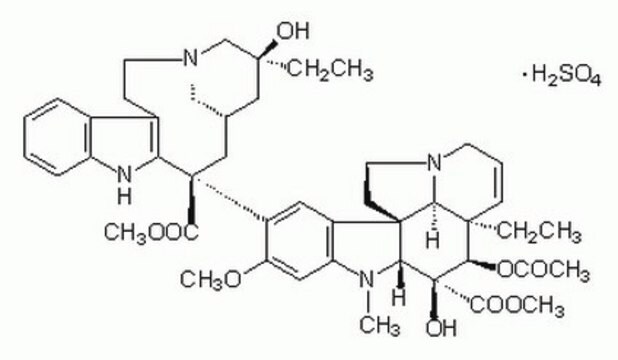
Vincristine sulfate salt
95.0-105.0% (HPLC), powder or crystals
Synonym(s):
22-Oxovincaleukoblastine sulfate salt, Leurocristine sulfate salt, VCR
About This Item
Recommended Products
biological source
Streptomyces roseosporus
Quality Level
assay
95.0-105.0% (HPLC)
form
powder or crystals
storage condition
(Keep container tightly closed in a dry and well-ventilated place. Keep in a dry place.)
color
white to light yellow
antibiotic activity spectrum
neoplastics
mode of action
protein synthesis | interferes
storage temp.
−20°C
SMILES string
OS(O)(=O)=O.CC[C@]1(O)CC2CN(CCc3c([nH]c4ccccc34)[C@@](C2)(C(=O)OC)c5cc6c(cc5OC)N(C=O)C7[C@](O)([C@H](OC(C)=O)[C@]8(CC)C=CCN9CC[C@]67C89)C(=O)OC)C1
InChI
1S/C46H56N4O10.H2O4S/c1-7-42(55)22-28-23-45(40(53)58-5,36-30(14-18-48(24-28)25-42)29-12-9-10-13-33(29)47-36)32-20-31-34(21-35(32)57-4)50(26-51)38-44(31)16-19-49-17-11-15-43(8-2,37(44)49)39(60-27(3)52)46(38,56)41(54)59-6;1-5(2,3)4/h9-13,15,20-21,26,28,37-39,47,55-56H,7-8,14,16-19,22-25H2,1-6H3;(H2,1,2,3,4)/t28-,37-,38+,39+,42-,43+,44+,45-,46-;/m0./s1
InChI key
AQTQHPDCURKLKT-PNYVAJAMSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
Analysis Note
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Oral - Muta. 2 - Repr. 2
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We presents an article on ABC Transporters and Cancer Drug Resistance
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service