SAE0156
Bone Alkaline Phosphatase from human bone osteosarcoma cells
lyophilized, ≥2000 u/mg protein
Synonym(s):
ALPL, BAP, Bone ALP, Ostase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352204
NACRES:
NA.77
Recommended Products
General description
Mammalian alkaline phosphatases are a group of membrane-bound glycoprotein isoenzymes that are attached to the cell membrane by a hydrophobic glycosyl-phosphatidylinositol (GPI) anchor.
The bone alkaline phosphatase isozyme is expressed from the gene for tissue nonspecific alkaline phosphatase which is also expressed in liver and kidney. These three isoforms form the majority of alkaline phosphatases circulating in serum. All three forms share the same amino acid sequence but have different carbohydrate and lipid modifications that provide unique properties according to the source tissue.
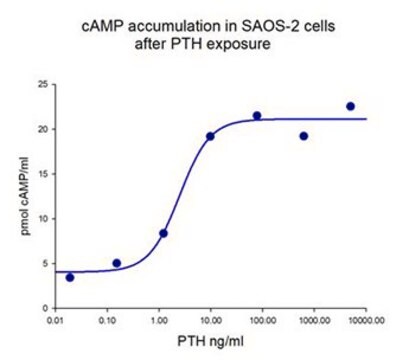
Bone alkaline phosphatase is synthesized by osteoblasts, involved in the calcification of bone matrix, but its precise role in bone formation process is still unknown. It is a highly specific marker of the bone-forming activity of osteoblasts. High activity of bone alkaline phosphatase is observed in serum in several bone diseases, such as Paget′s disease, osteoporosis, ricket disease, bone metastatic carcinoma, and others. Physiological bone growth can also contribute to increased levels of bone alkaline phosphatase in serum.
This product is purified from human osteosarcoma Saos-2 cells, an established cell line with high basal alkaline phosphatase activity. Before purification, alkaline phosphatase is released from the cell membrane by enzymatic cleavage of the GPI anchor.
The bone alkaline phosphatase isozyme is expressed from the gene for tissue nonspecific alkaline phosphatase which is also expressed in liver and kidney. These three isoforms form the majority of alkaline phosphatases circulating in serum. All three forms share the same amino acid sequence but have different carbohydrate and lipid modifications that provide unique properties according to the source tissue.
Bone alkaline phosphatase is synthesized by osteoblasts, involved in the calcification of bone matrix, but its precise role in bone formation process is still unknown. It is a highly specific marker of the bone-forming activity of osteoblasts. High activity of bone alkaline phosphatase is observed in serum in several bone diseases, such as Paget′s disease, osteoporosis, ricket disease, bone metastatic carcinoma, and others. Physiological bone growth can also contribute to increased levels of bone alkaline phosphatase in serum.
This product is purified from human osteosarcoma Saos-2 cells, an established cell line with high basal alkaline phosphatase activity. Before purification, alkaline phosphatase is released from the cell membrane by enzymatic cleavage of the GPI anchor.
Unit Definition
One unit will hydrolyze 1.0 μmole of p-nitrophenyl phosphate to p-nitrophenol and inorganic phosphate per minute at pH 9.8 at 37 °C.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service