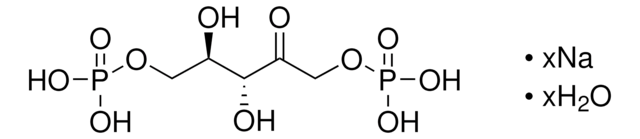
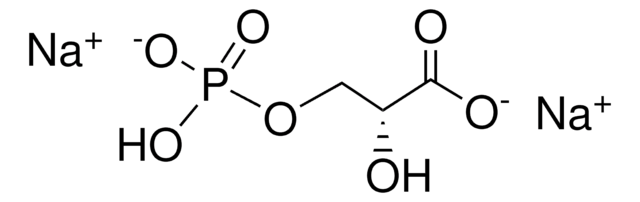
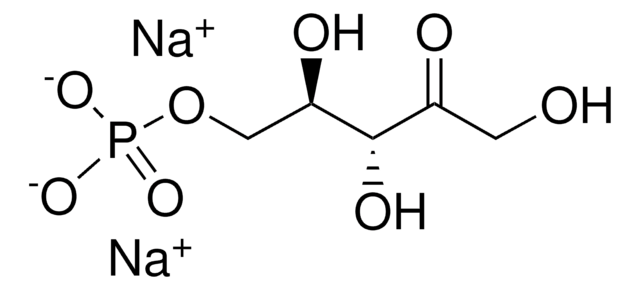
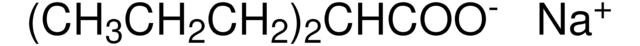R8000
D-Ribulose 1,5-Diphosphate Carboxylase from spinach
partially purified powder, 0.01-0.1 unit/mg solid
Synonym(s):
3-Phospho-D-glycerate carboxy-lyase(dimerizing), Rubisco
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
biological source
spinach
Quality Level
form
partially purified powder
specific activity
0.01-0.1 unit/mg solid
mol wt
557 kDa
storage temp.
−20°C
General description
D-Ribulose 1,5-Diphosphate Carboxylase (RUBISCO) amounts to 50% of the total spinach leaves associated soluble protein.
Exists as a 557 kDa hexadecamer composed of eight heavy chains each with a molecular weight of approximately 56 kDa and eight light chains of molecular weight 14 kDa. Each molecule contains one magnesium ion.
pH optimum: ~7.9.
KM for CO2: ~0.45 mM.
Ribulose diphosphate becomes inhibitory at concentrations exceeding 0.7 mM. Orthophosphate and ammonium sulfate are competitive inhibitors. 3-Phosphoglycerate is a noncompetitive inhibitor.
pH optimum: ~7.9.
KM for CO2: ~0.45 mM.
Ribulose diphosphate becomes inhibitory at concentrations exceeding 0.7 mM. Orthophosphate and ammonium sulfate are competitive inhibitors. 3-Phosphoglycerate is a noncompetitive inhibitor.
Application
D-Ribulose 1,5-Diphosphate Carboxylase from spinach has been used:
- as a test protein in pepsin digestion studies
- as an innocuous or non-hazardous protein sample to test its effect on human intestinal epithelial cell lines
- in isothermal titration calorimetry (ITC), and radiolabeled binding assays with abscisic acid
Biochem/physiol Actions
D-Ribulose 1,5-Diphosphate Carboxylase (RUBISCO) depends on Rubisco activase and chaperones for activation. It participates in plant photorespiration events by catalyzing the carboxylation and oxygenation of ribulose-1,5-bisphosphate. Abscisic acid inhibits the carboxylation activity of Rubisco.
Unit Definition
One unit will convert 1.0 μmole of D-RuDP and CO2 to 2.0 μmoles of D-3-phosphoglycerate per min at pH 7.8 at 25°C.
signalword
Danger
hcodes
pcodes
Hazard Classifications
Resp. Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yuji Suzuki et al.
Journal of experimental botany, 64(4), 1145-1152 (2013-01-26)
Rubisco gene expression was examined in detail in rice (Oryza sativa L.) leaves at different positions, i.e. expanding, mature, and senescent leaves. Rubisco small subunit (RBCS) synthesis and RBCS mRNA levels were maximal in expanding leaves and gradually became lower
Romain A Studer et al.
Proceedings of the National Academy of Sciences of the United States of America, 111(6), 2223-2228 (2014-01-29)
A well-known case of evolutionary adaptation is that of ribulose-1,5-bisphosphate carboxylase (RubisCO), the enzyme responsible for fixation of CO2 during photosynthesis. Although the majority of plants use the ancestral C3 photosynthetic pathway, many flowering plants have evolved a derived pathway
Libor Vítek et al.
Antioxidants (Basel, Switzerland), 11(11) (2022-11-12)
Oxidative stress and inflammation contribute significantly to atherogenesis. We and others have demonstrated that mildly elevated serum bilirubin levels protect against coronary and peripheral atherosclerosis, most likely due to the antioxidant and anti-inflammatory activities of bilirubin. The aim of the
Hiroyuki Ishida et al.
Biochimica et biophysica acta, 1837(4), 512-521 (2013-11-26)
Chloroplasts are the primary energy suppliers for plants, and much of the total leaf nitrogen is distributed to these organelles. During growth and reproduction, chloroplasts in turn represent a major source of nitrogen to be recovered from senescing leaves and
Ma Linglan et al.
Biological trace element research, 122(2), 168-178 (2008-01-15)
Characterized by a photocatalysis property, nanoanatase is closely related to the photosynthesis of spinach. It could not only improve light absorbance, transformation from light energy to electron energy, and active chemical energy, but also promote carbon dioxide (CO(2)) assimilation of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service