P1053
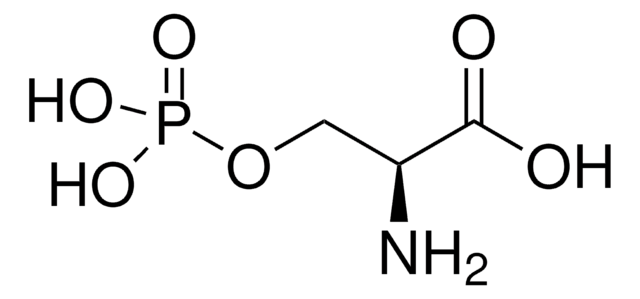
O-Phospho-L-threonine
≥98.0% (TLC)
Synonym(s):
(S)-2-Amino-3-hydroxybutanoic acid 3-phosphate, L-Threonine O-phosphate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C4H10NO6P
CAS Number:
Molecular Weight:
199.10
Beilstein/REAXYS Number:
1727078
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
Recommended Products
Product Name
O-Phospho-L-threonine,
assay
≥98.0% (TLC)
Quality Level
form
powder
color
white
storage temp.
−20°C
SMILES string
C[C@@H](OP(O)(O)=O)[C@H](N)C(O)=O
InChI
1S/C4H10NO6P/c1-2(3(5)4(6)7)11-12(8,9)10/h2-3H,5H2,1H3,(H,6,7)(H2,8,9,10)/t2-,3+/m1/s1
InChI key
USRGIUJOYOXOQJ-GBXIJSLDSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
O-Phospho-L-threonine is an amino acid derivative.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Christian B Oehlenschlæger et al.
Frontiers in plant science, 8, 2005-2005 (2017-12-13)
PSY1R is a leucine-rich repeat (LRR) receptor-like kinase (RLK) previously shown to act as receptor for the plant peptide hormone PSY1 (peptide containing sulfated tyrosine 1) and to regulate cell expansion. PSY1R phosphorylates and thereby regulates the activity of plasma
H Christian Reinhardt et al.
Nature reviews. Molecular cell biology, 14(9), 563-580 (2013-08-24)
Coordinated progression through the cell cycle is a complex challenge for eukaryotic cells. Following genotoxic stress, diverse molecular signals must be integrated to establish checkpoints specific for each cell cycle stage, allowing time for various types of DNA repair. Phospho-Ser/Thr-binding
Shijie Wang et al.
Acta neuropathologica communications, 5(1), 86-86 (2017-11-24)
Missense mutations in the leucine-rich repeat kinase 2 (LRRK2) gene can cause late-onset Parkinson disease (PD). LRRK2 mutations increase LRRK2 kinase activities that may increase levels of LRRK2 autophosphorylation at serine 1292 (pS1292) and neurotoxicity in model systems. pS1292-LRRK2 protein
Lisa N Glass et al.
PLoS pathogens, 13(7), e1006515-e1006515 (2017-07-29)
We have previously shown that the Mycobacterium tuberculosis universal stress protein Rv2623 regulates mycobacterial growth and may be required for the establishment of tuberculous persistence. Here, yeast two-hybrid and affinity chromatography experiments have demonstrated that Rv2623 interacts with one of
Dennis Wong et al.
Scientific reports, 8(1), 155-155 (2018-01-11)
Protein phosphorylation plays a key role in Mycobacterium tuberculosis (Mtb) physiology and pathogenesis. We have previously shown that a secreted protein tyrosine phosphatase, PtpA, is essential for Mtb inhibition of host macrophage acidification and maturation, and is a substrate of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service