N6287
Nutlin-3
≥98% (HPLC), powder, Mdm2 antagonist
Synonym(s):
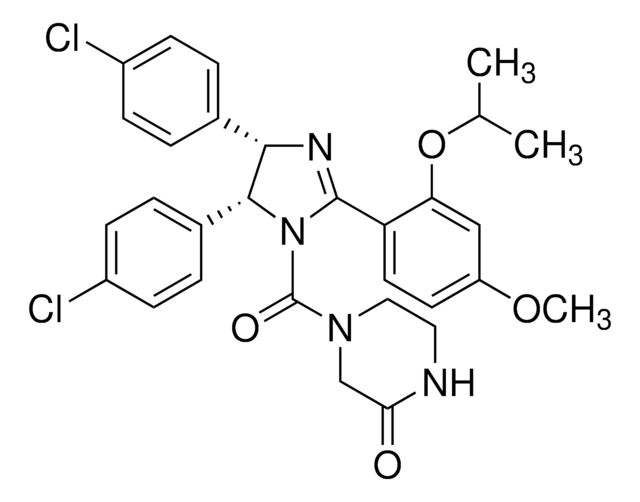
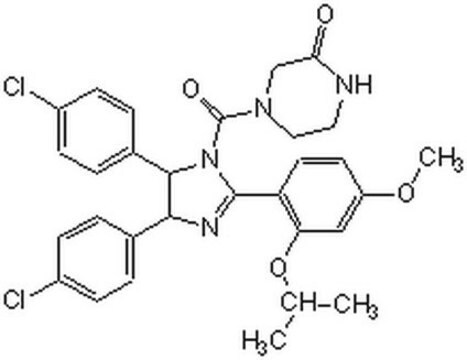
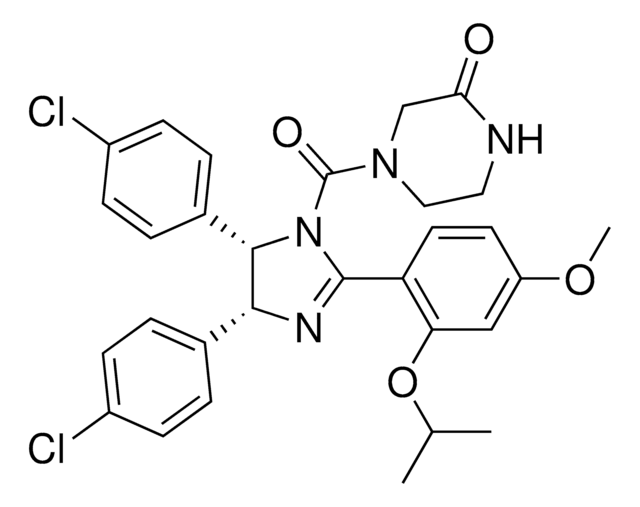
(±)-4-[4,5-Bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxy-phenyl)-4,5-dihydro-imidazole-1-carbonyl]-piperazin-2-one
About This Item
Recommended Products
Product Name
Nutlin-3, ≥98% (HPLC), powder
ligand
nutlin-3
assay
≥98% (HPLC)
form
powder
reaction suitability
reagent type: ligand
solubility
DMSO: 20 mg/mL
H2O: insoluble
originator
Roche
shipped in
wet ice
storage temp.
−20°C
SMILES string
O=C(N1CC(NCC1)=O)N2C(C3=CC=C(Cl)C=C3)C(C4=CC=C(Cl)C=C4)N=C2C5=C(OC(C)C)C=C(OC)C=C5
InChI
1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m1/s1
InChI key
BDUHCSBCVGXTJM-IZLXSDGUSA-N
Application
- as a drug to stimulate p53 functions in gene transfer experiment
- to inject worms to verify the prevalent role of translationally controlled tumor protein (TCTP) in posterior amputated E. eugeniae
- as a p53 activator in cyclotherapy studies
- as an mdm2 inhibitor to know its effect on p53 levels, cleaved caspase 3 and Poly (ADP-ribose) polymerase (PARP) cleavage
Biochem/physiol Actions
Features and Benefits
Other Notes
Analysis Note
Legal Information
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Apoptosis, or programmed cell death (PCD), is a selective process for the removal of unnecessary, infected or transformed cells in various biological systems. As it plays a role in the homeostasis of multicellular organisms, apoptosis is tightly regulated through two principal pathways by a number of regulatory and effector molecules.
n proliferating cells, the cell cycle consists of four phases. Gap 1 (G1) is the interval between mitosis and DNA replication that is characterized by cell growth. Replication of DNA occurs during the synthesis (S) phase, which is followed by a second gap phase (G2) during which growth and preparation for cell division occurs. Together, these three stages comprise the interphase phase of the cell cycle. Interphase is followed by the mitotic (M) phase.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service