L4919
Lysozyme from chicken egg white
BioUltra, lyophilized powder, ≥98% (SDS-PAGE), ≥40,000 units/mg protein
Synonym(s):
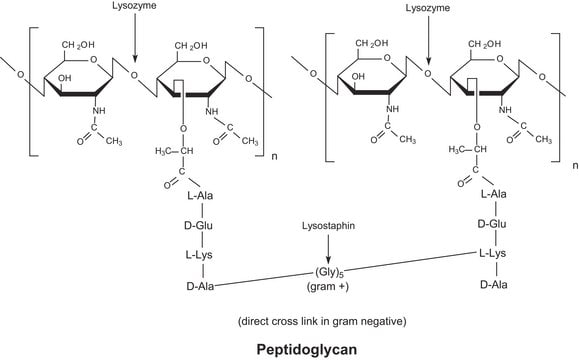
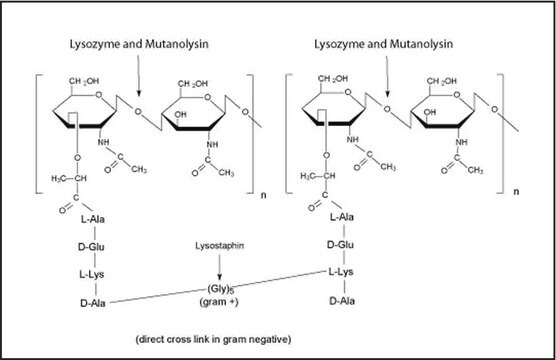
Mucopeptide N-acetylmuramoylhydrolase, Muramidase
About This Item
Recommended Products
biological source
chicken egg white
Quality Level
grade
for molecular biology
product line
BioUltra
assay
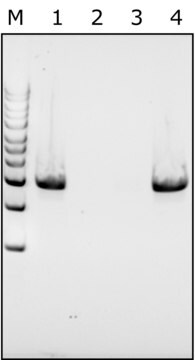
≥98% (SDS-PAGE)
form
lyophilized powder
specific activity
≥40,000 units/mg protein
mol wt
single-chain 14.3 kDa
composition
Protein, ≥90%
technique(s)
cell based assay: suitable
suitability
suitable for cell lysis
application(s)
cell analysis
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Application
- in dielectric spectroscopy studies of dynamics of protein
- as a control to measure the human lysozyme activity
- as a supplement in soaking solution to treat lenses
Biochem/physiol Actions
The enzyme is active over a broad pH range (6.0 to 9.0). At pH 6.2, maximal activity is observed over a wider range of ionic strengths (0.02 to 0.100 M) than at pH 9.2 (0.01 to 0.06 M).
Features and Benefits
- Highly purified by repeated crystallization and dialysis
- Each lot is use-tested for isolation of plasmid DNA from E. coli
Unit Definition
Preparation Note
signalword
Danger
hcodes
pcodes
Hazard Classifications
Resp. Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Separation of Ribonuclease A from bovine pancreas, Type I-A, powder, ≥60% RNase A basis (SDS-PAGE), ≥50 Kunitz units/mg protein; α-Chymotrypsinogen A from bovine pancreas, essentially salt-free, lyophilized powder; Cytochrome c from bovine heart, ≥95% based on Mol. Wt. 12,327 basis; Lysozyme from chicken egg white, lyophilized powder, protein ≥90 %, ≥40,000 units/mg protein
Separation of Ribonuclease A from bovine pancreas, Type I-A, powder, ≥60% RNase A basis (SDS-PAGE), ≥50 Kunitz units/mg protein; α-Chymotrypsinogen A from bovine pancreas, essentially salt-free, lyophilized powder; Cytochrome c from bovine heart, ≥95% based on Mol. Wt. 12,327 basis; Lysozyme from chicken egg white, lyophilized powder, protein ≥90 %, ≥40,000 units/mg protein
Separation of Ribonuclease A from bovine pancreas, Type I-A, powder, ≥60% RNase A basis (SDS-PAGE), ≥50 Kunitz units/mg protein; α-Chymotrypsinogen A from bovine pancreas, essentially salt-free, lyophilized powder; Cytochrome c from bovine heart, ≥95% based on Mol. Wt. 12,327 basis; Lysozyme from chicken egg white, lyophilized powder, protein ≥90 %, ≥40,000 units/mg protein
Separation of Ribonuclease A from bovine pancreas, Type I-A, powder, ≥60% RNase A basis (SDS-PAGE), ≥50 Kunitz units/mg protein; α-Chymotrypsinogen A from bovine pancreas, essentially salt-free, lyophilized powder; Cytochrome c from bovine heart, ≥95% based on Mol. Wt. 12,327 basis; Lysozyme from chicken egg white, lyophilized powder, protein ≥90 %, ≥40,000 units/mg protein
Protocols
This enzymatic rate determination may be used for Lysozyme products. It is not to be used to assay recombinant or insoluble Lysozyme on agarose.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service