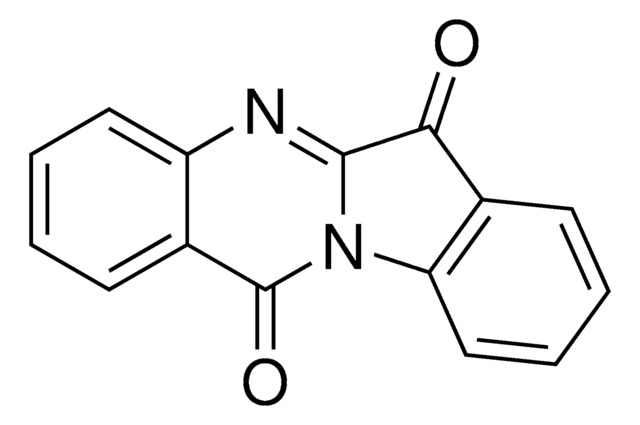
I0404
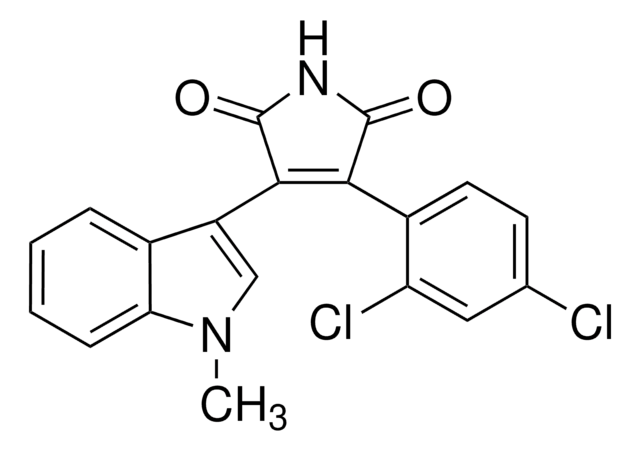
Indirubin-3′-oxime
≥98% (HPLC), solid
Synonym(s):
Indirubin-3′-monoxime
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H11N3O2
CAS Number:
Molecular Weight:
277.28
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
assay
≥98% (HPLC)
form
solid
mol wt
277.28
solubility
DMSO: >10 mg/mL
H2O: insoluble
storage temp.
room temp
SMILES string
O\N=C1\C(Nc2ccccc12)=C3\C(=O)Nc4ccccc34
InChI
1S/C16H11N3O2/c20-16-13(9-5-1-3-7-11(9)18-16)15-14(19-21)10-6-2-4-8-12(10)17-15/h1-8,17,21H,(H,18,20)/b15-13-,19-14+
InChI key
HBDSHCUSXQATPO-BGBJRWHRSA-N
Application
Indirubin-3′-oxime has been used in the inhibition of glycogen synthase kinase 3 in human monocytic cell line, THP-1.
Biochem/physiol Actions
Indirubin-3′-oxime is a cyclin-dependent kinase inhibitor which functions by competing with ATP for binding to the catalytic subunit; exhibits antiproliferative activity leading to G2/M arrest in many cell lines and G1/S arrest in Jurkat cells.
Indirubin-3′-oxime mediates apoptosis in Jurkat T cells and has anti-tumor functionality. Indirubin-3′-oxime inhibits Y box binding protein 1 (YB1) translocation, contributing to anticancer functionality. Indirubin-3′-oxime decreases expression of estrogen-related receptor γ (ERRγ) and peroxisome proliferator-activated receptor-γ co-activator-1α (PGC1α) in human neuroblastoma, leading to cell cycle arrest and mitochondrial dysfunction.
Features and Benefits
This compound is featured on the CDKs and GSK-3 pages of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Indirubin-3'-oxime induces mitochondrial dysfunction and triggers growth inhibition and cell cycle arrest in human neuroblastoma cells.
Liao XM and Leung KN
Oncology Reports, 29(1), 371-379 (2013)
Dynamic modulation of innate immune response by varying dosages of LPS in human monocytic cells.
Morris M, et al.
The Journal of Biological Chemistry, 289(31), 21584-21590 (2014)
Mechanism responsible for inhibitory effect of indirubin 3?-oxime on anticancer agent-induced YB-1 nuclear translocation in HepG2 human hepatocellular carcinoma cells.
Tanaka T, et al.
Experimental Cell Research, 370(2), 454-460 (2018)
Semi-synthesis of indirubin-3′-oxime from Strobilanthes cusia leaves, its acute and sub-chronic toxicity, in vitro and in vivo antitumor activity in Lewis lung carcinoma bearing mice.
Cuong NM, et al.
Journal of Pharmacognosy and Phytochemistry, 5(4), 205-205 (2016)
Ming-Yang Lee et al.
European journal of pharmacology, 839, 57-65 (2018-09-30)
Cholangiocarcinoma (CCA) is one of the most serious of all cancers and a major public health problem. CCA is an extremely invasive cancer, and the survival rate for CCA patients is only 24 months after diagnosis. Although surgery and chemotherapy
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service