D9766
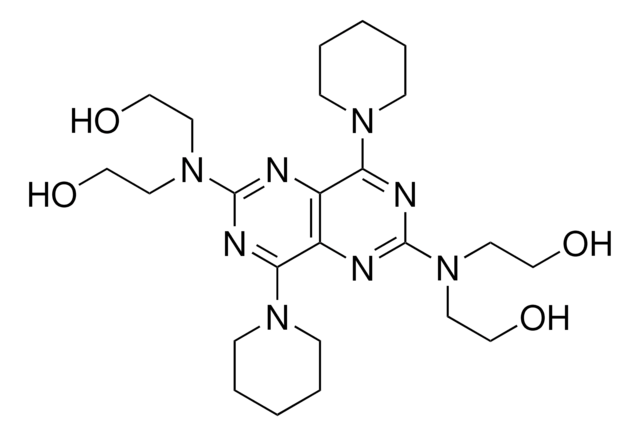
Dipyridamole
≥98% (HPLC), powder, phosphodiesterase V inhibitor
Synonym(s):
NSC 515776, NSC 619103
About This Item
Recommended Products
Product Name
Dipyridamole, ≥98% (HPLC)
assay
≥98% (HPLC)
form
powder
color
yellow
mp
165-166 °C (lit.)
solubility
DMSO: soluble
ethanol: soluble
originator
Boehringer Ingelheim
storage temp.
room temp
SMILES string
OCCN(CCO)c1nc(N2CCCCC2)c3nc(nc(N4CCCCC4)c3n1)N(CCO)CCO
InChI
1S/C24H40N8O4/c33-15-11-31(12-16-34)23-26-20-19(21(27-23)29-7-3-1-4-8-29)25-24(32(13-17-35)14-18-36)28-22(20)30-9-5-2-6-10-30/h33-36H,1-18H2
InChI key
IZEKFCXSFNUWAM-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544) , PDE10A(10846) , PDE1A(5136) , PDE1B(5153) , PDE1C(5137) , PDE2A(5138) , PDE3A(5139) , PDE3B(5140) , PDE4A(5141) , PDE4B(5142) , PDE4C(5143) , PDE4D(5144) , PDE5A(8654) , PDE6A(5145) , PDE6B(5158) , PDE6C(5146) , PDE6D(5147) , PDE6G(5148) , PDE6H(5149) , PDE7A(5150) , PDE7B(27115) , PDE8A(5151) , PDE8B(8622) , PDE9A(5152) , SLC29A1(2030)
mouse ... Slc29a1(63959)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- to perform in vitro growth inhibition assay(40)
- to determine its ability to prevent uterine myometrial contractions(41)
- to determine its effects on nicotinamide adenine dinucleotide (NAD+)-induced increase in intracellular adenosine triphosphate (ATP) levels(42)
- to prevent nicotinamide riboside (NR)-induced axonal protection(43)
Biochem/physiol Actions
Features and Benefits
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Cyclic nucleotides, including cyclic AMP (cAMP), cyclic GMP (cGMP) and cyclic ADP-ribose, have been extensively studied as second messengers of intracellular events initiated by activation of GPCRs. cAMP modifies cell function in all eukaryotic cells, principally through the activation of cAMP-dependent protein kinase (PKA), but also through cAMP-gated ion channels and guanine nucleotide exchange factors directly activated by cAMP.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service