CG201
cGMP Enzyme Immunoassay Kit
sufficient for 96 assays
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12161503
NACRES:
NA.32
Recommended Products
usage
sufficient for 96 assays
Quality Level
technique(s)
ELISA: suitable
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... PRKG1(5592)
General description
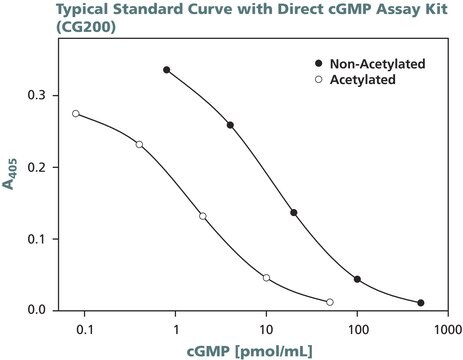
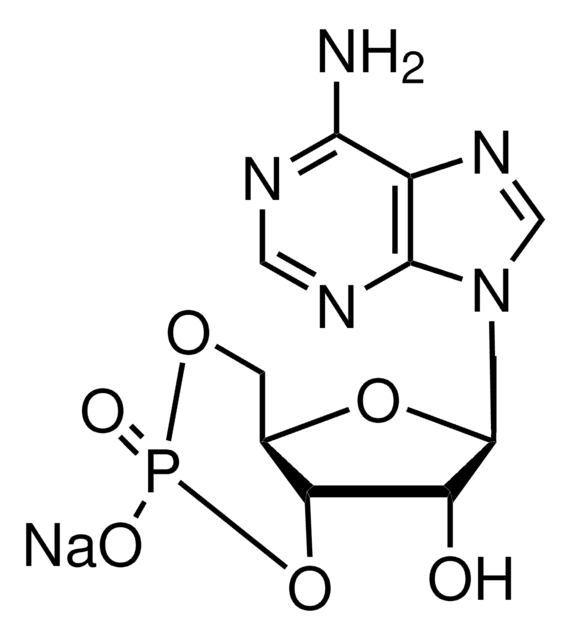
cGMP Enzyme Immunoassay Kit is non-radioactive, competitive immunoassay for the quantitation of cyclic guanine monophosphate (cGMP) in biological fluids (serum, plasma, urine and saliva), tissue culture media samples or in samples containing very low concentrations of cyclic nucleotides. This kit uses a polyclonal antibody to cGMP to competitively bind cGMP or cGMP that has been covalently linked to an alkaline phosphatase molecule. The immunoassay is performed in a 96 well plate coated with anti-rabbit IgG antibody. The colored end product, produced by the addition of substrate to the wells, is read at 405 nm on a multiwell plate reader. The intensity of the color is inversely proportional to the concentration of cGMP present in the well.
Application
Non-radioactive, competitive immunoassay for the quantitation of cGMP in biological fluids (serum, plasma, urine and saliva), tissue culture media samples, or in samples containing very low concentrations of cyclic nucleotides. This kit uses a polyclonal antibody to cGMP to competitively bind cGMP or cGMP that has been covalently linked to an alkaline phosphatase molecule. The assay is performed in a 96 well plate coated with anti-rabbit IgG antibody. The colored end product, produced by the additon of substrate to the wells, is read at 405 nm on a multiwell plate reader. The intensity of the color is inversely proportional to the concentration of cGMP present in the well.
cGMP Enzyme Immunoassay Kit has been used for the quantitative determination of cyclic guanine monophosphate (cGMP) concentration in parasite tissues and mouse aortic artery homogenates. It has also been used to assess the guanylate cyclase activity.
Kit Components Only
Product No.
Description
- Acetic Anhydride 1 x 1
- Assay Buffer 2 1 x 30
- cGMP-Alkaline Phosphatase Conjugate 1 x 5
- cGMP Assay Layout Sheet 1 x 1
- cGMP EIA Antibody Rabbit Anti-cGMP 1 x 5
- 5 Cycle Logit-Log Paper 1 x 1
- Cyclic GMP Standard 1 x 0.5
- Goat Anti-Rabbit IgG Coated 96 Well Microtiter Plate 1 ea
- p-Nitrophenyl Phosphate Substrate Solution 1 x 20
- Plate Sealer 1 ea
- Stop Solution 1 x 5
- Triethylamine 1 x 2
- Wash Buffer Concentrate 1 x 30
See All (13)
signalword
Danger
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
flash_point_f
12.2 °F - closed cup
flash_point_c
-11 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Inhibition of MPO (Myeloperoxidase) Attenuates Endothelial Dysfunction in Mouse Models of Vascular Inflammation and Atherosclerosis
Cheng D, et al.
Arteriosclerosis, Thrombosis, and Vascular Biology, 39(7), 1448-1457 (2019)
Identification of a novel Arabidopsis thaliana nitric oxide-binding molecule with guanylate cyclase activity in vitro
Mulaudzi T, et al.
Febs Letters, 585(17), 2693-2697 (2011)
Genistein from Flemingia vestita (Fabaceae) enhances NO and its mediator (cGMP) production in a cestode parasite, Raillietina echinobothrida
Das B, et al.
Parasitoloty, 134(10), 1457-1463 (2007)
David Cheng et al.
Arteriosclerosis, thrombosis, and vascular biology, 39(7), 1448-1457 (2019-05-03)
Objective- Inflammation-driven endothelial dysfunction initiates and contributes to the progression of atherosclerosis, and MPO (myeloperoxidase) has been implicated as a potential culprit. On release by circulating phagocytes, MPO is thought to contribute to endothelial dysfunction by limiting NO bioavailability via
Chard, T.
An Introduction to Radioimmunoassay and Related Techniques (1990)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service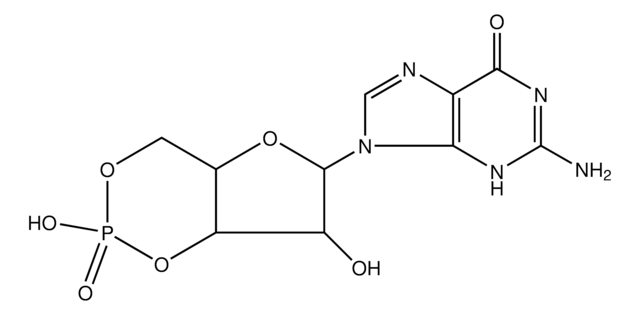


![Western Blocking Reagent, Solution solution, sufficient for 10 blots (11921673001 [100 cm2]), sufficient for 60 blots (11921681001 [100 cm2])](/deepweb/assets/sigmaaldrich/product/images/352/091/ef743cea-ccd8-44f1-8f3b-dec5a1e4f5d1/640/ef743cea-ccd8-44f1-8f3b-dec5a1e4f5d1.jpg)





