C0253
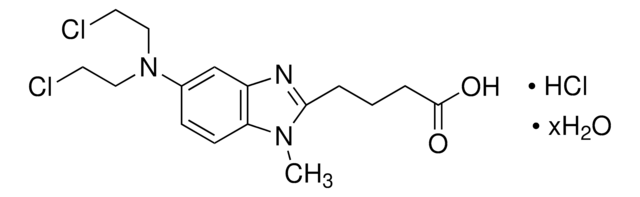
Chlorambucil
Synonym(s):
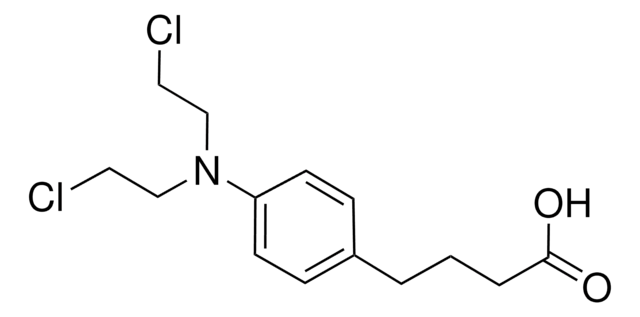
4-(4-[Bis(2-chloroethyl)amino]phenyl)butyric acid, 4-[Bis(2-chloroethyl)amino]benzenebutyric acid
About This Item
Recommended Products
form
powder
Quality Level
storage temp.
2-8°C
SMILES string
OC(=O)CCCc1ccc(cc1)N(CCCl)CCCl
InChI
1S/C14H19Cl2NO2/c15-8-10-17(11-9-16)13-6-4-12(5-7-13)2-1-3-14(18)19/h4-7H,1-3,8-11H2,(H,18,19)
InChI key
JCKYGMPEJWAADB-UHFFFAOYSA-N
Gene Information
human ... CYP2D6(1565)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- as an alkylating/chemotherapeutic agent to study its anti-inflammatory effects on amyloidogenesis in mice
- as a positive control to determine the cytotoxicity in murine leukemic monocyte macrophages
- as an adenine-specific DNA-alkylating reagent in the construction of multifunctional conjugate (8950A-Chb(Cl/OH) for mutation-specific DNA alkylation in HeLa S3 cells
Biochem/physiol Actions
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Carc. 1B - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service