B4527
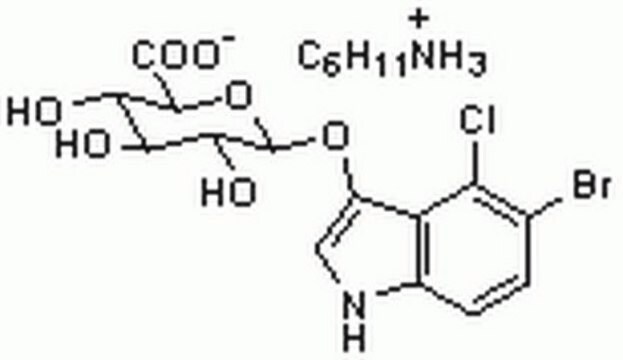
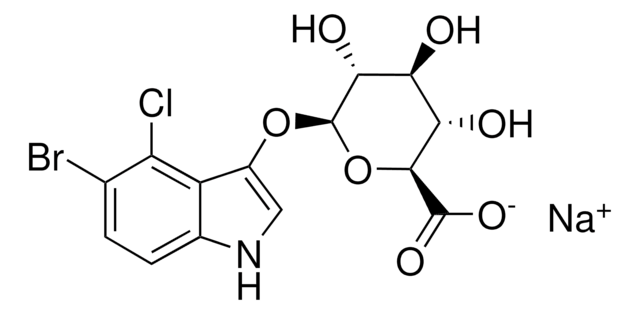
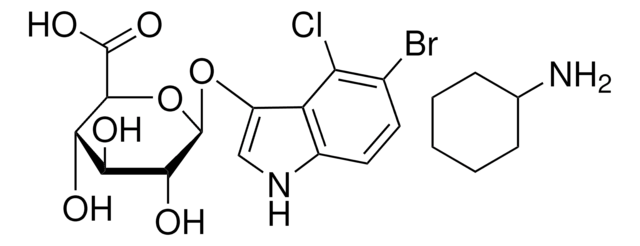
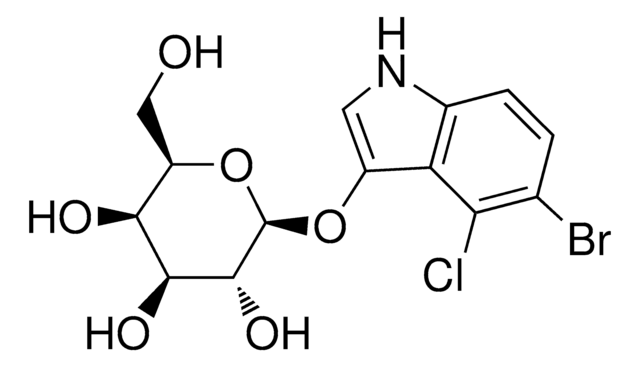
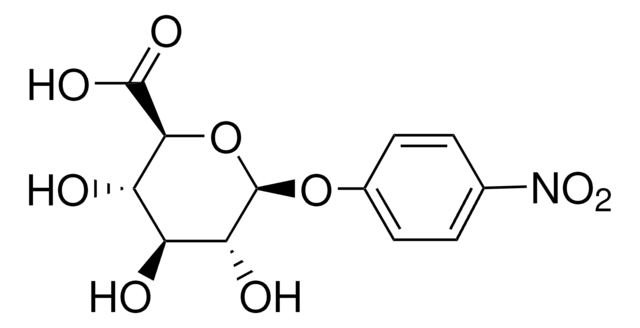
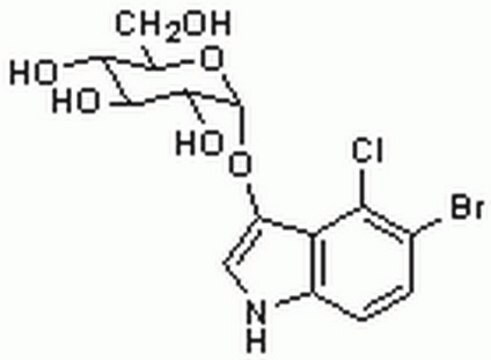
5-Bromo-4-chloro-3-indolyl β-D-glucopyranoside
chromogenic, ≥97%, powder
Synonym(s):
X-Glc, X-glucoside
About This Item
Recommended Products
Product Name
5-Bromo-4-chloro-3-indolyl β-D-glucopyranoside, ≥97%
Quality Level
assay
≥97%
form
powder
solubility
DMF: 50 mg/mL, clear, colorless to faintly yellow
storage temp.
−20°C
SMILES string
OC[C@H]1O[C@@H](Oc2c[nH]c3ccc(Br)c(Cl)c23)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C14H15BrClNO6/c15-5-1-2-6-9(10(5)16)7(3-17-6)22-14-13(21)12(20)11(19)8(4-18)23-14/h1-3,8,11-14,17-21H,4H2/t8-,11-,12+,13-,14-/m1/s1
InChI key
OPIFSICVWOWJMJ-LNNRFACYSA-N
Looking for similar products? Visit Product Comparison Guide
Substrates
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Today, diverse studies report the benefits of probiotics, such as inhibitory effects on pathogens, aid in the management or prevention of chronic intestinal inflammatory diseases or atopic syndromes, and support to the immune system. Potential beneficial applications abound, researchers continue to evaluate the effictiveness and clarify the mechanisms of action of probiotics.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service