17793
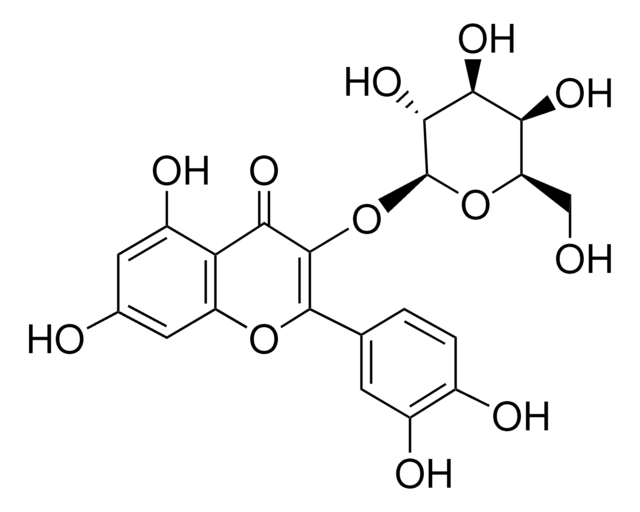
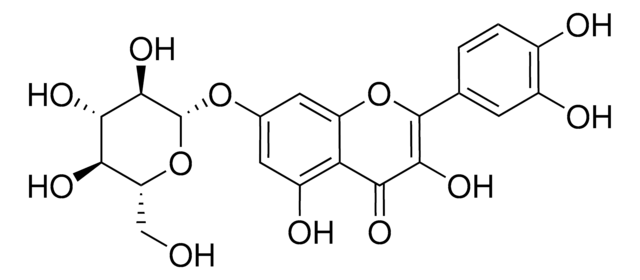
Quercetin 3-β-D-glucoside
≥90% (HPLC)
Synonym(s):
3,3′,4′,5,7-Pentahydroxyflavone 3-β-glucoside, Isoquercitrin
About This Item
Recommended Products
Quality Level
assay
≥90% (HPLC)
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
−20°C
SMILES string
OC[C@H]1O[C@@H](OC2=C(Oc3cc(O)cc(O)c3C2=O)c4ccc(O)c(O)c4)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-27,29-30H,6H2/t13-,15-,17+,18-,21+/m1/s1
InChI key
OVSQVDMCBVZWGM-QSOFNFLRSA-N
Gene Information
mouse ... Hexa(15211)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as a dietary flavonoid supplement to check its binding capacity with human small ubiquitin-related modifier 1 (SUMO1) protein using surface plasmon resonance (SPR)
- as an inhibitor for Escherichia coli adenosine triphosphate (ATP) synthase
- as an anti-aggregation agent to test its activity against β-amyloid, green fluorescent protein (GFP), and chymotrypsinogen proteins
Biochem/physiol Actions
Packaging
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
HPLC Analysis of Polyphenols in Nero d'Avola Red Wine on Discovery® HS C18 (UV 280 nm)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service