T176
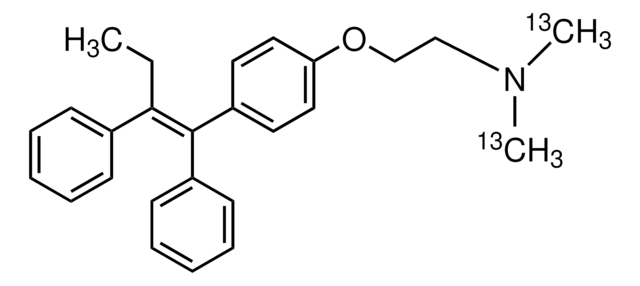
4-Hydroxytamoxifen
analytical standard, (E) and (Z) isomers (50:50)
Synonym(s):
4-(1-[4-(Dimethylaminoethoxy)phenyl]-2-phenyl-1-butenyl)phenol, 4-OHT, cis/trans-4-Hydroxytamoxifen
About This Item
Recommended Products
grade
analytical standard
Quality Level
form
powder
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
solubility
ethanol: 20 mg/mL
antibiotic activity spectrum
neoplastics
application(s)
forensics and toxicology
pharmaceutical (small molecule)
format
neat
mode of action
enzyme | inhibits
SMILES string
CC\C(c1ccccc1)=C(/c2ccc(O)cc2)c3ccc(OCCN(C)C)cc3.CC\C(c4ccccc4)=C(\c5ccc(O)cc5)c6ccc(OCCN(C)C)cc6
InChI
1S/2C26H29NO2/c2*1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h2*5-17,28H,4,18-19H2,1-3H3/b26-25+;26-25-
InChI key
ZJLDABGSDWXVGE-BDSXMVAQSA-N
Gene Information
human ... ESR1(2099) , ESR2(2100) , ESRRG(2104) , IL6(3569)
rat ... Ar(24208) , Esr1(24890) , Esr2(25149)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Determination of tamoxifen and its three metabolites from dried blood spot (DBS) discs by ultra-high performance liquid chromatography-electrospray ionization-tandem mass spectrometry (UHPLC-ESI-MS/MS)
- Simultaneous estimation of tamoxifen, 4-hydroxytamoxifen, and endoxifen from DBS samples by UHPLC-MS/MS
- Development and validation of a non-aqueous capillary electrophoretic (NACE) method coupled with capacitively coupled contactless conductivity detection (C4D) to analyze tamoxifen and its three main metabolites after their liquid-liquid extraction (LLE) from human plasma samples obtained from breast cancer patients
- Multi-residue analysis of human plasma samples to quantify tamoxifen and its degradation products using the non-aqueous capillary electrophoretic (NACE) method combined with UV-detection
- Combined detection of tamoxifen and centchroman, along with their degradation products in human plasma samples by LC-ESI-MS/MS
Biochem/physiol Actions
Other Notes
signalword
Warning
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service