62118
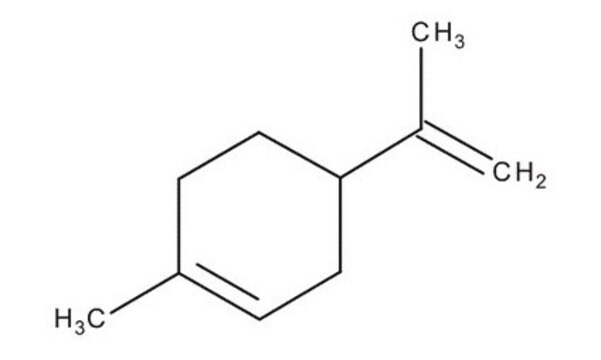
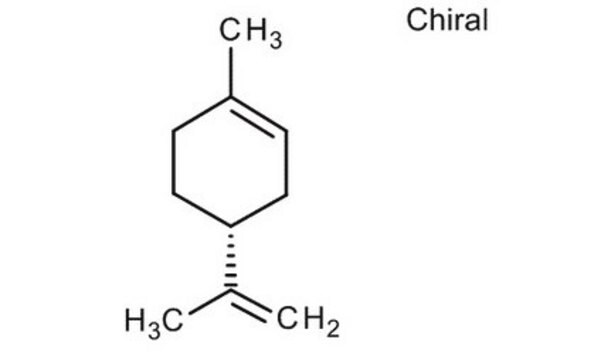
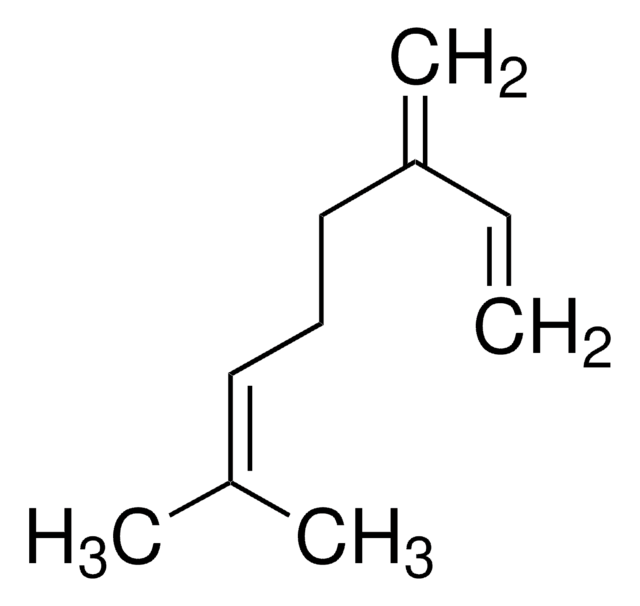
(R)-(+)-Limonene
analytical standard
Synonym(s):
(+)-p-Mentha-1,8-diene, (+)-Carvene, (R)-4-Isopropenyl-1-methyl-1-cyclohexene
About This Item
Recommended Products
grade
analytical standard
Quality Level
vapor density
4.7 (vs air)
vapor pressure
<3 mmHg ( 14.4 °C)
assay
≥99.0% (sum of enantiomers, GC)
optical activity
[α]20/D +115.5±1°, c = 10% in ethanol
shelf life
limited shelf life, expiry date on the label
expl. lim.
6.1 %
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.473 (lit.)
n20/D 1.473
bp
176-177 °C (lit.)
density
0.842 g/mL at 20 °C (lit.)
application(s)
agriculture
cleaning products
cosmetics
environmental
flavors and fragrances
food and beverages
personal care
format
neat
storage temp.
2-8°C
SMILES string
CC(=C)[C@@H]1CCC(C)=CC1
InChI
1S/C10H16/c1-8(2)10-6-4-9(3)5-7-10/h4,10H,1,5-7H2,2-3H3/t10-/m0/s1
InChI key
XMGQYMWWDOXHJM-JTQLQIEISA-N
Looking for similar products? Visit Product Comparison Guide
General description
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Application
- Analysis of alcoholic beverages for the determination of (R)-(+)-limonene by differential pulse voltammetry (DPV) using two novel electrochemical sensors
- Simultaneous determination of limonene and linalool in 10 perfume product samples by two-dimensional high-performance liquid chromatographic (HPLC) method combined with electrospray ionization tandem mass spectrometry (ESI-MS/MS)
- Determination of the enantiomeric composition of volatile chiral compounds, commonly present in three plant species from the Citrus genus by multidimensional gas chromatography (MDGC) coupled to mass spectrometry (MS)
- Multi-residue analysis of volatiles and fatty acids found in wild and cultivated fennel samples by a single extraction method and gas chromatographic-flame ionization detection (GC-FID)
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Flam. Liq. 3 - Skin Irrit. 2 - Skin Sens. 1
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
123.8 °F - closed cup
flash_point_c
51 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-(+)-Limonene, purum, ≥98.0% (sum of enantiomers, GC); Geranyl tiglate; α-Terpineol, natural, ≥96%, FCC, FG; Geranyl formate; α-Pinene
-3,7-Dimethyl-2,6-octadien-1-ol; Neral; Geraniol; Geranial; Undecanal; Citronellyl acetate; Neryl acetate; 3,7-Dimethyl-2,6-octadienyl acetate; 1-Tetradecene; Tetradecane; α-Bisabolol
-β-Farnesene; α-Huµlene; Germacrene D; (+)-Valencene; Bicyclogermacrene; (+)-δ-Cadinene
-Cymene; 2,5-Dimethylpyrrole; Acetoin, ≥96%, FCC, FG; 2,5-Dimethylpyrazine; 2,6-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Dimethylpyrazine; 4-Heptanone; 3-Ethylpyridine; 2,3,5-Trimethylpyrazine; Furfural; Pyrrole; Furfuryl acetate; Linalool; Linalyl acetate; 5-Methylfurfural; γ-Butyrolactone; 2-Acetyl-1-methylpyrrole; Furfuryl alcohol; 2-Acetylpyrrole; Pyrrole-2-carboxaldehyde
Related Content
Gas chromatography separates volatile compounds in the gas phase, applied in various industries for quality control.
Gas chromatography separates volatile compounds in the gas phase, applied in various industries for quality control.
Gas chromatography is a common analytic technique used to separate and analyze volatile compounds in the gas phase. GC is applied in many industries for quality control, and to identify and/or quantify compounds in a mixture.
Gas chromatography is a common analytic technique used to separate and analyze volatile compounds in the gas phase. GC is applied in many industries for quality control, and to identify and/or quantify compounds in a mixture.
Chromatograms
suitable for GCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service