23128
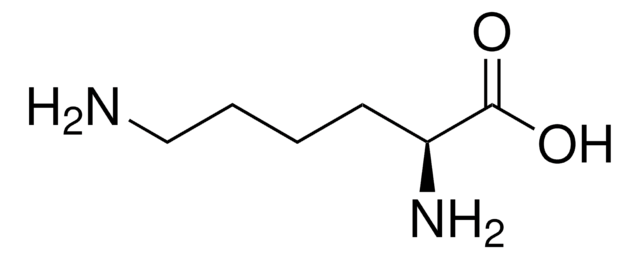
L-Lysine
analytical standard
Synonym(s):
(S)-2,6-Diaminocaproic acid
About This Item
Recommended Products
grade
analytical standard
Quality Level
assay
≥95.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
analyte chemical class(es)
amino acids, peptides, proteins
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
impurities
≤2.0% water
color
white to off-white
mp
215 °C (dec.) (lit.)
application(s)
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
format
neat
SMILES string
NCCCC[C@H](N)C(O)=O
InChI
1S/C6H14N2O2/c7-4-2-1-3-5(8)6(9)10/h5H,1-4,7-8H2,(H,9,10)/t5-/m0/s1
InChI key
KDXKERNSBIXSRK-YFKPBYRVSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Recommended products
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Separation of L-Alanine; Glycine; L-Valine; L-Leucine; L-Isoleucine; L-Proline; L-Methionine; L-Serine; L-Threonine; L-Phenylalanine; L-Aspartic acid; L-4-Hydroxyproline; L-Cysteine; L-Glutamic acid; L-Asparagine; L-Lysine; L-Glutamine; L-Histidine; L-Tyrosine; L-Tryptophan; L-Cystine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service