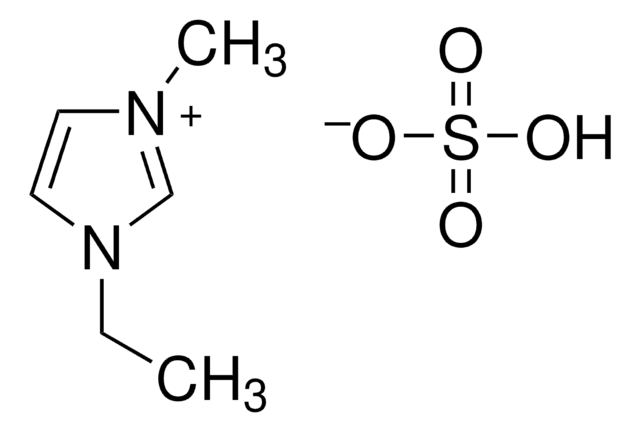
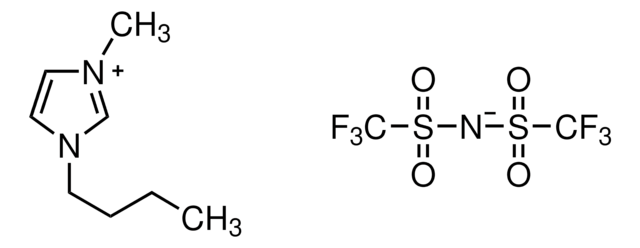
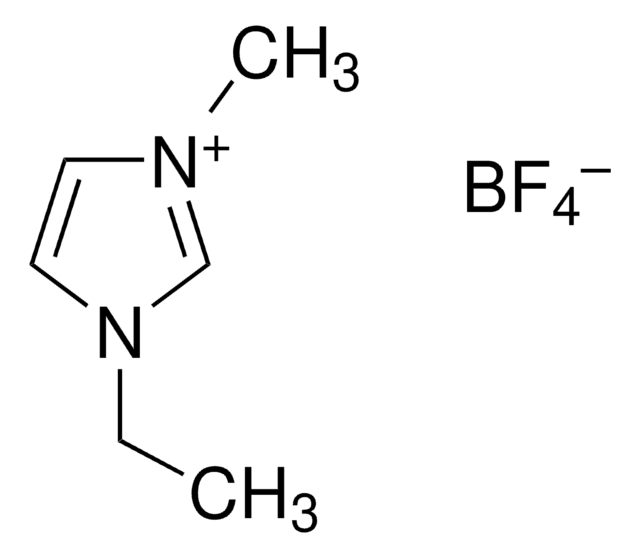
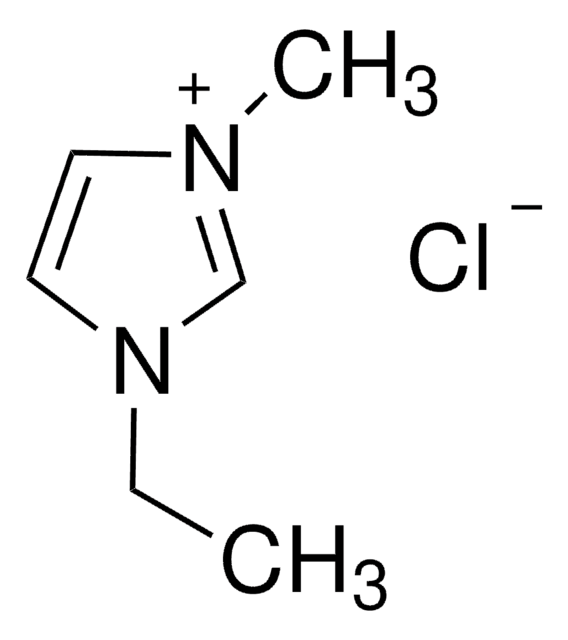
11291
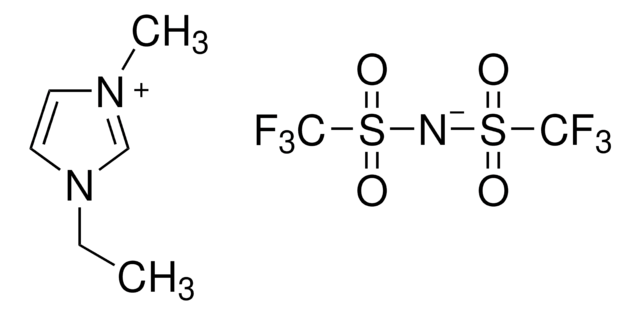
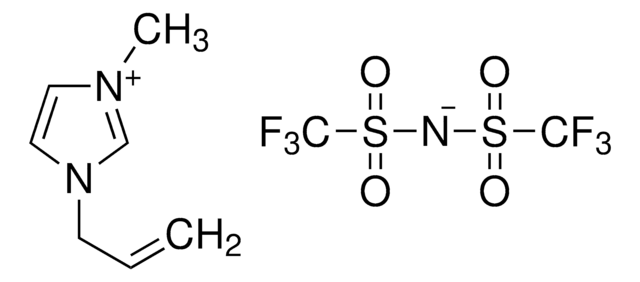
1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide
≥97.0% (NMR)
Synonym(s):
EMIM BTI, EMIM TFSI, EMIMIm
About This Item
Recommended Products
Quality Level
assay
≥97.0% (NMR)
form
liquid
impurities
<0.5% water
mp
≥−15 °C (lit.)
SMILES string
CCn1cc[n+](C)c1.FC(F)(F)S(=O)(=O)[N-]S(=O)(=O)C(F)(F)F
InChI
1S/C6H11N2.C2F6NO4S2/c1-3-8-5-4-7(2)6-8;3-1(4,5)14(10,11)9-15(12,13)2(6,7)8/h4-6H,3H2,1-2H3;/q+1;-1
InChI key
LRESCJAINPKJTO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Physical form
Other Notes
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 1
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Over the past decade, Ionic Liquids have attracted much interest for their use as non-aqueous electrolytes in electrochemical applications. In this context, their conductivity as well as their electrochemical stability are the most important physical properties.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service