LEU-RO
Roche
Leupeptin
powder, from synthetic, =96.5%
Synonym(s):
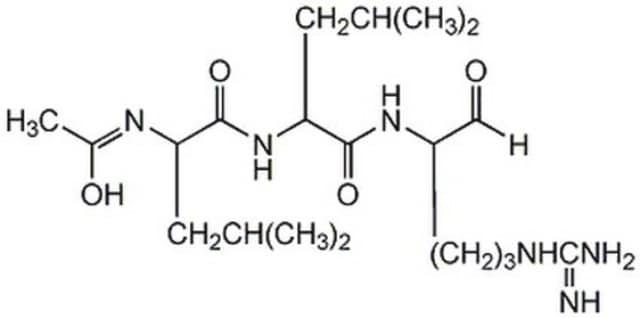
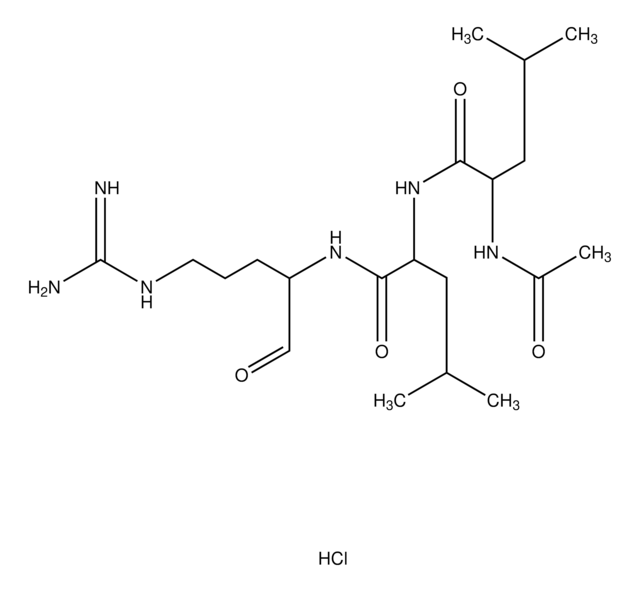
Leupeptin hemisulfate salt, Acetyl-Leu-Leu-Arg-al, N-Acetyl-L-leucyl-L-leucyl-L-argininal hemisulfate salt
About This Item
Recommended Products
biological source
synthetic
Quality Level
description
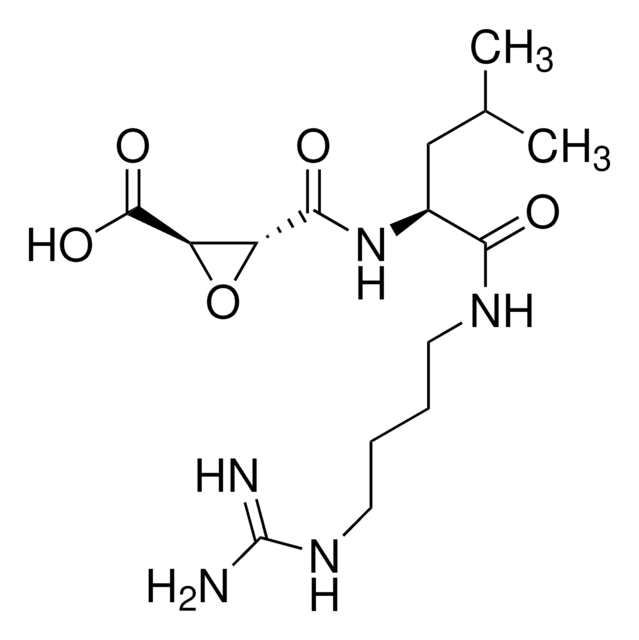
Ac-Leu-Leu-argininal x 1/2 H2SO4, synthetic
assay
96.5%
form
powder
mol wt
Mr 475.6
packaging
pkg of 100 mg (11529048001)
pkg of 25 mg (11017128001)
pkg of 5 mg (11017101001)
pkg of 50 mg (11034626001)
manufacturer/tradename
Roche
solubility
H2O: 50 mg/mL
shipped in
wet ice
storage temp.
2-8°C
SMILES string
OS(O)(=O)=O.CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C=O.CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C=O
InChI
1S/2C20H38N6O4.H2O4S/c2*1-12(2)9-16(24-14(5)28)19(30)26-17(10-13(3)4)18(29)25-15(11-27)7-6-8-23-20(21)22;1-5(2,3)4/h2*11-13,15-17H,6-10H2,1-5H3,(H,24,28)(H,25,29)(H,26,30)(H4,21,22,23);(H2,1,2,3,4)/t2*15-,16-,17-;/m00./s1
InChI key
CIPMKIHUGVGQTG-VFFZMTJFSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Specificity
Not affected are α-, β-, γ- and δ- chymotrypsin, pepsin, cathepsin D, elastase, renin, and thermolysin.
Application
Note: To check other protease inhibitors, try our Protease Inhibitor Set including Antipain Dihydrochloride, Aprotinin, Bestatin, Chymostatin, E-64, EDTA-Na2, Leupeptin, Pefabloc SC, Pepstatin, and Phosphoramidon.
Biochem/physiol Actions
Features and Benefits
Synthetic, white powder
Quality
Preparation Note
Comparison of working concentrations of pefabloc with leupeptin and PMSF is in files.
Working solution: Solvent is recommended in distilled water.
Highly soluble in water (1 mg/ml), methanol, ethanol, acetic acid, dimethyl formamide and dimethyl sulfoxide.
Poorly soluble in acetone, chloroform, ethyl ether and n-hexane.
Storage conditions (working solution): -15 to -25 °C
In aqueous solution leupeptin is stable for 1 month at 2 to 8 °C or for at least 6 months at - 15 to -25 °C, stored under nitrogen. For best results, freeze the dissolved inhibitor in aliquots and avoid repeated thawing.
Reconstitution
Analysis Note
Other Notes
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service