10105031001
Roche
β-Galactosidase
from E. coli overproducer
Synonym(s):
β-galactosidase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
Escherichia coli
Quality Level
form
suspension
mol wt
540 kDa
packaging
pkg of 1,500 U
manufacturer/tradename
Roche
concentration
5.00 mg/mL
technique(s)
activity assay: suitable
optimum pH
7
suitability
suitable for UV spectrophotometry and general use
application(s)
life science and biopharma
shipped in
wet ice
storage temp.
2-8°C
General description
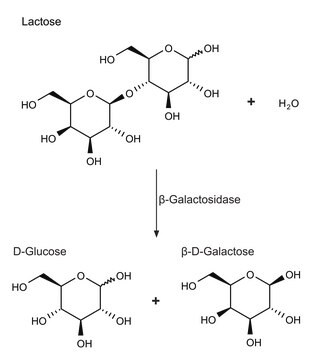
β-Galactosidase or β-D-Galactoside galactohydrolase is basically a tetramer made up of identical four polypeptide chains, each constituting 1023 amino acids. It is very specific for D-galactose and requires K+ or Na+ and Mg2+ to be fully active. β-Galactosidase enzyme with an oxygen glycosidic bond catalyzes reactions with β-d-galactopyranosides.
At 37 °C with 2-nitrophenyl-β-D-galactoside as the substrate, approximately 30 U/mg at 25 °C with lactose as the substrate; standardized with BSA.
Specificity
Cleaves terminal galactose residues that are β1,4-linked to a monosaccharide, oligosaccharide, or glycopeptide.
Application
β-galactosidase has been used in enzyme-linked immunosorbent assay (ELISA). Use β-Galactosidase to produce a calibration curve in enzymatic assays.
Quality
Contaminants: <0.01% GIDH, GPT, LDH, MDH, and oxaloacetate decarboxylase, each
Physical form
Suspension, in 3.2 M ammonium sulfate solution, pH approximately 6, crystalline
Preparation Note
The ammonium sulfate preparation is stable at 2 to 8 °C until the expiration date printed on the label. Use directly for most applications, e.g., quantitation of lactose.
In the absence of ammonium sulfate, solutions of β-galactosidase should be stabilized with Mg2+ (89 mM) and a thiol reagent (1mM β-mercaptoethanol, 1 mM dithiothreitol) [Beutler, 1984]. The thiol slows the formation of enzyme dimers resulting from intramolecular disulfide bridges.
Activator: K (50 mM) is required for activation (lactose hydrolysis).
Na (50 mM) is also an activator, particularly for hydrolysis of 2-nitrophenyl-β-D-galactopyranoside.
In the absence of ammonium sulfate, solutions of β-galactosidase should be stabilized with Mg2+ (89 mM) and a thiol reagent (1mM β-mercaptoethanol, 1 mM dithiothreitol) [Beutler, 1984]. The thiol slows the formation of enzyme dimers resulting from intramolecular disulfide bridges.
Activator: K (50 mM) is required for activation (lactose hydrolysis).
Na (50 mM) is also an activator, particularly for hydrolysis of 2-nitrophenyl-β-D-galactopyranoside.
Other Notes
For life science research only. Not for use in diagnostic procedures.
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 1
flash_point_f
No data available
flash_point_c
No data available
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Intradermal Electroporation of Naked Replicon RNA Elicits Strong Immune Responses
Johansson D X, et al.
PLoS ONE, 7(1), e29732-e29732 (2012)
Douglas H Juers et al.
Protein science : a publication of the Protein Society, 21(12), 1792-1807 (2012-09-27)
This review provides an overview of the structure, function, and catalytic mechanism of lacZ β-galactosidase. The protein played a central role in Jacob and Monod's development of the operon model for the regulation of gene expression. Determination of the crystal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service