TZHALA205
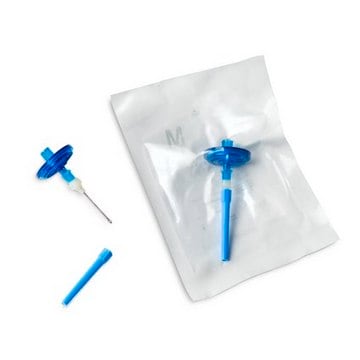
Steritest® NEO Device
For liquids in ampoules or collapsible bags., Blue base canister with a single-needle adapter., pkg of 10 blisters in 2 bags of 5 blisters per box, double packed
Synonym(s):
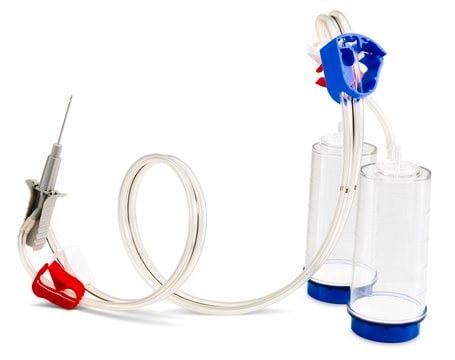
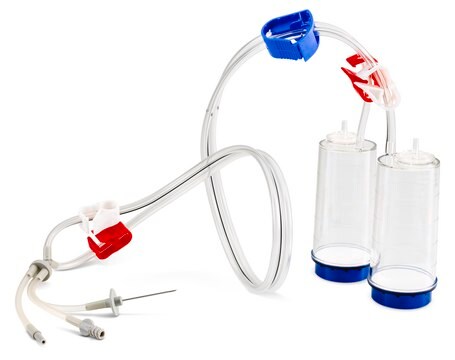
Blue Base Steritest® NEO device for sterility testing
About This Item
Recommended Products
material
Nylon 66 adapter (for needle)
PVC tubing (double lumen)
mixed cellulose esters (MCE) membrane
plain filter
stainless steel (for needle)
styrene-acrylonitrile (SAN) (for canister)
Quality Level
agency
EP (2.6.1)
JP (4.06)
USP 71
sterility
sterile; γ-irradiated
feature
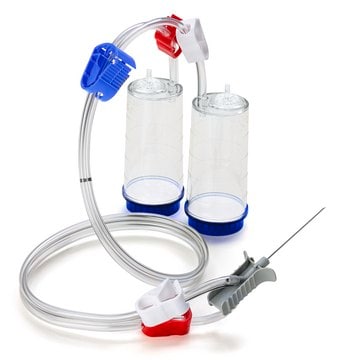
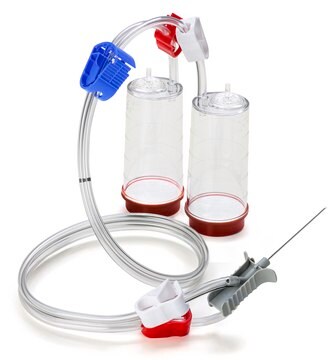
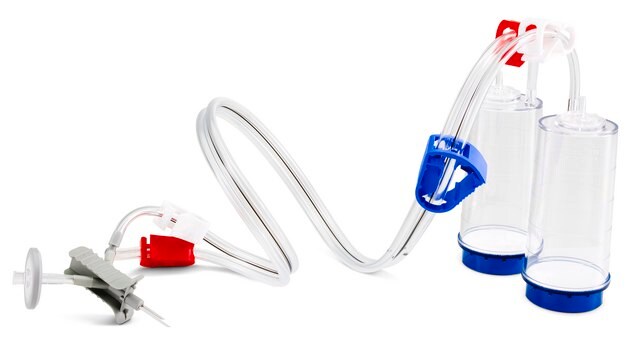
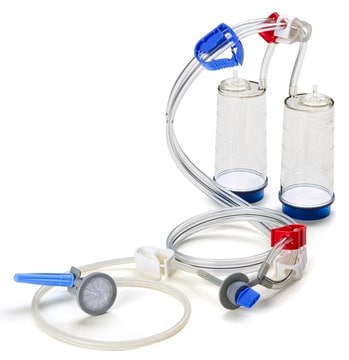
Blue base canister with a single-needle adapter.
manufacturer/tradename
Steritest®
packaging
pkg of 10 blisters in 2 bags of 5 blisters per box, double packed
parameter
120 mL sample volume (graduation marks at 25, 50, 75 and 100 mL)
3.1 bar max. inlet pressure (45 psi) at 25 °C
45 °C max. temp.
tubing L
850 mm
color
blue Canister Base
matrix
MF-Millipore™
pore size
0.45 μm pore size
input
liquid
sample type pharmaceutical(s)
application(s)
pharmaceutical
sterility testing
compatibility
For liquids in ampoules or collapsible bags.
shipped in
ambient
General description
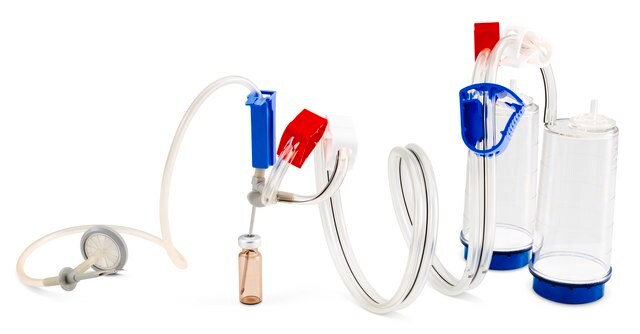
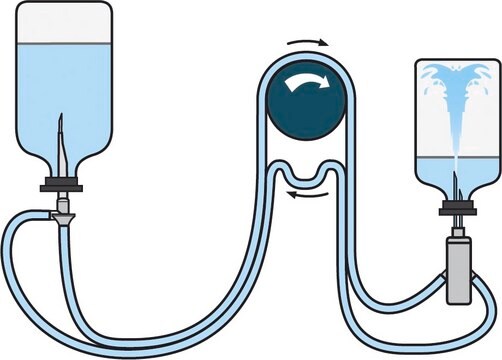
Steritest® NEO is a membrane filtration device for sterility testing of filterable pharmaceutical products. The device simplifies every aspect of testing, from handling to traceability. The closed system ensures that pharmaceutical products are never exposed to the environment, minimizes false positives, and offers the highest levels of quality and reliability. This test system offers an optimized and fully regulatory compliant testing process when used with the Steritest® Symbio pump, specific accessories and high-quality culture media and rinsing fluids. The device is gamma sterilized and double packed for the quick transfer into sterility testing environments, simplifying decontamination procedures and saving time. A single needle adapter enables an easy access to ampoules or collapsible bags. A separate vent needle is included for the transfer of the test product, culture media or rinsing buffer. The blue based canisters indicate mixed cellulose ester (MCE) membrane, which provides an optimal filtration flow rate for standard products. The new Velax® cutting clamp allows a safe and clean disconnection of the tubing and is readily mounted on all Steritest® NEO devices.
Application
Legal Information
Configured for
Related product
Required but not provided
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Regulatory compliant membrane filtration sterility testing devices to ensure the safety of your pharmaceutical products.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service