STBMCTM12
Clear Fluid Thioglycollate Medium
ready-to-use, bottle volume 100 mL , filling Volume
Synonym(s):
CTM, Clear FTM, Clear Thioglycollate Broth, Fluid Thioglycollate Medium (Clear), Thioglyc. Med. (FTM) clear, Thioglycollate B. 0.1l clear
About This Item
Recommended Products
agency
in accordance with EP 2.6.1
in accordance with JP 4.06
in accordance with USP 71
Quality Level
sterility
sterile; autoclaved
form
liquid
shelf life
12 mo.
feature
closure type screw cap with septum
ready-to-use
packaging
bottle of
bottle capacity
125 mL
bottle volume
100 mL , filling Volume
input
sample type pharmaceutical(s)
pH
7.1
application(s)
cosmetics
food and beverages
pharmaceutical
sterility testing
compatibility
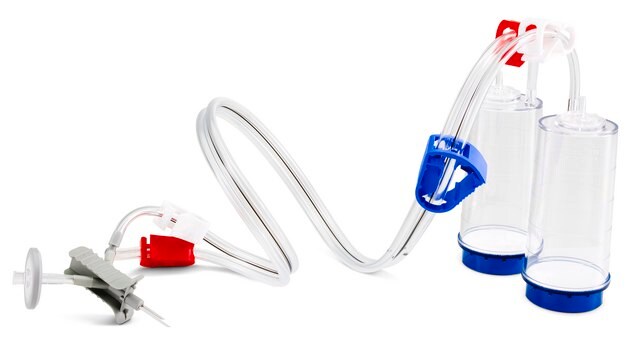
for use with Steritest® Symbio FLEX Pump Kit, 2 media (SYMBFLE01)
for use with Steritest® Symbio ISL Pump Kit, 2 media (SYMBISL01)
for use with Steritest® Symbio LFH Pump Kit (SYMBLFH01)
for use with Steritest®
shipped in
ambient
storage temp.
2-25°C
suitability
aerobic bacteria
nonselective for
General description
Application
Bioburden testing
Components
Specifications
Legal Information
comparable product
recommended
related product
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Direct Inoculations Method for Sterility Testing Media in various volumes by Merck. Solutions for pharmaceutical and cosmetics industries.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service